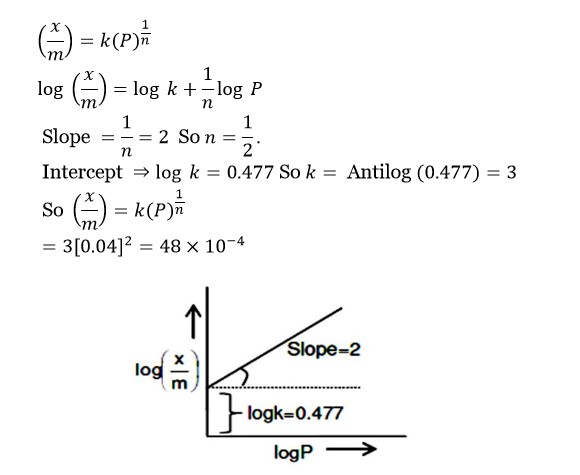
Surface Chemistry
Get insights from 216 questions on Surface Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Surface Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
For the coagulation of a negative sol flocculating power is
Al³? > Ba²? > Na?
But for coagulating a positive sol the flocculating power will be
PO? ³? > SO? ²? > Cl?
New answer posted
5 months agoContributor-Level 9
Metal sulphide sols are negatively charged while metal oxide sols are positively charged sols. So, CdS is negative while TiO? is positively charged sol.
New answer posted
5 months agoContributor-Level 10
Colloidal particles are small enough to pass through an ordinary filter but can be stopped by an ultrafilter paper due to their specific particle size range.
New answer posted
5 months agoContributor-Level 10
100 mol of KBr is doped with 10? mol of SrBr?
Therefore, 1 mol of KBr contains 10? mol of SrBr?
Molar mass of KBr = 119 g/mol .
119 g of KBr contains 10? mol of SrBr?
1 g of KBr contains (10? / 119) mol of SrBr?
Each Sr²? ion introduced creates one cation vacancy to maintain electrical neutrality.
Number of cation vacancies = (moles of SrBr? ) * (Avogadro's number)
Number of vacancies = (10? / 119) * (6.023 * 10²³) = 5.06 * 10¹?
The answer provided in the document is "5 (Rounded off)", which likely refers to the coefficient 5.06.
New answer posted
5 months agoContributor-Level 10
Solid sol is a colloidal system consisting of a gas dispersed in a solid, e.g., pumice stone, foam rubber.
New answer posted
5 months agoContributor-Level 10
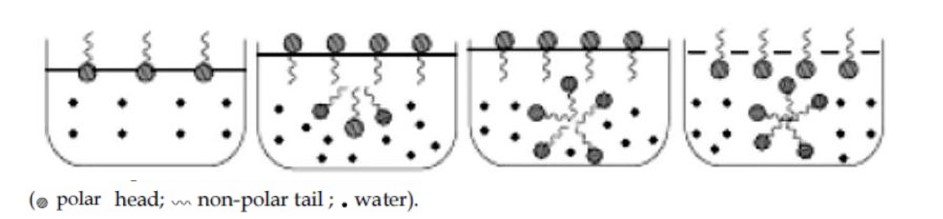
At CMC, the particles cluster together through lyophobic end to form associated colloid called micelle. Further all lyophilic ends (polar head) get projected towards water.
New answer posted
5 months agoContributor-Level 9
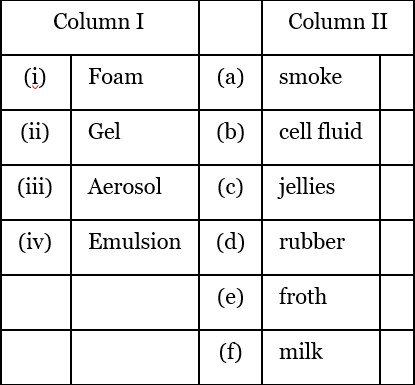
(i) Foam - Froth (e)
(ii) Gel - Jellies (c)
(iii) Aerosol - smoke (a)
(iv) Emulsion - milk (f)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers