Surface Chemistry
Get insights from 216 questions on Surface Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Surface Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 9
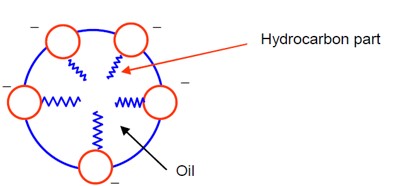
[Diagram of a micelle formed by sodium stearate in oil]
Spherical micelles forms and hydrocarbon part are water repelling and attracted by oily part.
New answer posted
5 months agoContributor-Level 10
Phase | Medium | Colloid Type |
| (1) Cheese | liquid | solid | Gel |
| (2) Pumice stone | gas | solid | Solid sol |
| (3) Hair cream | liquid | liquid | Emulsion |
| (4) Cloud | liquid | gas | Aerosol |
New answer posted
5 months agoContributor-Level 10
PV = nRT
1 * V =
V = 2.4 litre
Vol of O2 adsorbed per gm = 2.4 / 1.2 = 2 litre
New answer posted
5 months agoContributor-Level 10
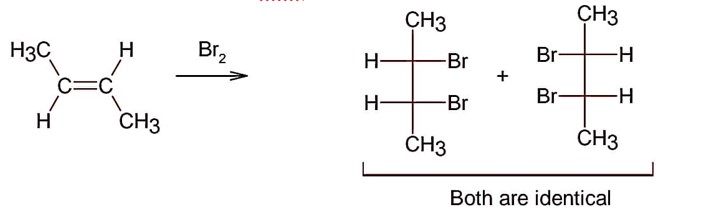
Electrophilic addition of bromine to an alkene is anti-addition, in which cis-alkene gives two enantiomers and trans – alkene gives meso form
Here; trans-but-2-ene will give meso products
New answer posted
5 months agoThe nature of charge on resulting colloidal particles when FeCl3 is added to excess of hot water is:
New answer posted
5 months agoContributor-Level 10
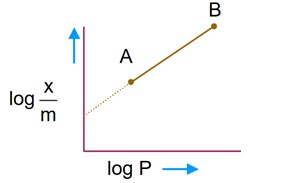
Freundlich adsorption isotherm
Comparing with y = mx + C
so, slope of given graph is
New answer posted
5 months agoContributor-Level 10
Freundlich adsorption Isotherm
At moderate pressure ;
So,
New answer posted
5 months agoContributor-Level 10
Viscosity of the hydrophilic sol is always higher than H2O, because dispersed phase particle attracted by dispersion medium.
New answer posted
6 months agoContributor-Level 10
When AgNO3 is added to Kl solution, precipitate of Agl is formed which adsorb I- ion from
Dispersion medium to give negatively charged sol
Agl/l-- negatively charged sol.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers