Surface Chemistry
Get insights from 216 questions on Surface Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Surface Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Factual
(A) Magnalium contain Al and Mg
(B) Bell metal ⇒ Cu and Sn
(C) Gun metal ⇒ Cu, Sn and Zn
(D) Chrome steel ⇒ Fe and Cr.
New answer posted
5 months agoContributor-Level 10
Fe acts a catalyst and Mo acts as promoter in Haber's process.
New answer posted
5 months agoContributor-Level 10
Number of mol of A reacted = 1*10? = 10?
Number of molecule of A = 10? * 6 *10²³
Quantum efficiency = (6 *10¹? ) / (1.2 *10¹? ) = 0.50
New answer posted
5 months agoContributor-Level 10
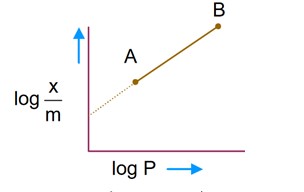
Freundlich adsorption isotherm
Comparing with y = mx + C
so, slope of given graph is
New answer posted
5 months agoContributor-Level 10
SO? is adsorbed more than H? on activated charcoal as critical temperature of SO? is higher than H? As higher the critical temperature, easier is liquifaction of gas and more is adsorption of gas on charcoal.
New answer posted
5 months agoContributor-Level 10
Keq. = ( [C] [D])/ ( [A] [B])
Keq = (10 * 6)/ (2 * 3) = 10
ΔG° = −2.303RTlog Keq. = −2.303 (2) (300) (log 10)
= −1381.8cal (? R = 2cal/molk)
New answer posted
5 months agoContributor-Level 10
When the reactants and the catalyst are in different phases, then the catalysis is known as heterogenous catalysis.
1. N? (g) + 3H? (g) - (Fe (s)-> 2NH? (g) (Heterogenous catalysis)
2. 2SO? (g) + O? (g) - (NO (g)-> 2SO? (g) (Homogenous catalysis)
3. C? H? O? (aq) + H? O (l) → C? H? O? (aq) + C? H? O? (aq) (Homogenous catalysis)
4. NO (g) + O? (g) → NO? (g) + O? (g)
New answer posted
5 months agoContributor-Level 10
Aspirin and paracetamol belongs to the class of non-narcotic analgesic.
Morphine and heroin are narcotic analgesics.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers