Thermodynamics
Get insights from 325 questions on Thermodynamics, answered by students, alumni, and experts. You may also ask and answer any question you like about Thermodynamics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
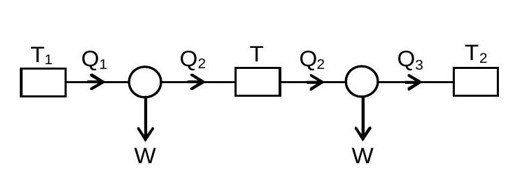
η_A = W_A/Q? = 1 - T/T?
η_B = W_B/Q? ' = 1 - T? /T
W_A = Q? (1 - T/T? ) = Q? - Q?
W_B = Q? ' (1 - T? /T) = Q? /2 (1 - T? /T)
Given W_A = W_B
Q? (1 - T/T? ) = (Q? /2) (1 - T? /T)
Q? (T? /T) (1-T/T? ) = (Q? /2) (1-T? /T)
(T? /T - 1) = (1/2) (1-T? /T)
2T? /T - 2 = 1 - T? /T
2T? /T + T? /T = 3
T = (2T? +T? )/3
New answer posted
6 months agoContributor-Level 10
Degrees of freedom (f) = 3 translational + 3 rotational + (2 * 4) vibrational = 14
γ = 1 + 2/f = 1 + 2/14 = 8/7
W = nR? T/ (γ-1) = (1 * 8.3 * (310-300)/ (8/7 - 1) = (83)/ (1/7) = 581 J
As W is positive, work is done by the gas. The solution says W<0, work done on the gas. This implies? T is negative. The question states temperature rises, so work is done on the gas. W = -582J.
New answer posted
6 months agoContributor-Level 10
H? O (l) → H? O (g)
ΔH° = ΔU° + ΔngRT
ΔH° - ΔU° = ΔngRT
= 1 * 8.31 * 373
= 3099.63 J/mol
= 30.9963 * 10² J/mol
≈ 31 * 10² J/mol
New answer posted
6 months agoContributor-Level 10
-dT/dt = K [T - Ts]
- (61-59)/4 = K [ (61+59)/2 - 30]
-0.5 = K [60 - 30] = 30K
So, K = -1/60 min? ¹
Again - (51-49)/t = K [ (51+49)/2 - 30]
-2/t = (-1/60) [50-30] = -20/60 = -1/3
t = 6 min
New answer posted
6 months agoContributor-Level 10
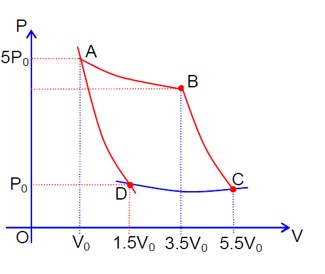
A→B & D→C (isothermal process)
So, TA = TB & TD = TC. Now B→C & D→A (adiabatic process)
|WBC| = nR/ (γ-1) (TB - TC)
|WAD| = nR/ (γ-1) (TA - TD) = nR/ (γ-1) (TB - TC)
∴ |WBC| = |WAD|
New answer posted
6 months agoContributor-Level 10
η = 1 - T? /T?
1/6 = 1 - T? /T? (i)
2η = 1/3 = 1 - (T? -62)/T? (ii)
By (i) and (ii)
(T? /T? ) = 5/6
1/3 = 1 - (T? /T? ) + 62/T? = 1 - 5/6 + 62/T?
1/3 = 1/6 + 62/T?
1/6 = 62/T?
T? = 62 * 6 = 372K = 99°C
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers