Alcohol Phenol And Ethers
Get insights from 261 questions on Alcohol Phenol And Ethers, answered by students, alumni, and experts. You may also ask and answer any question you like about Alcohol Phenol And Ethers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(ii) Assertion and reason both are wrong statements.
Explanation: Correct assertion: Phenol on treatment with bromine water can form 2,4,6- tribromophenol
Correct reason: In water, phenol gives phenoxide ions which activate the ring towards electrophilic substitution reaction.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(iii) Assertion is a correct statement but reason is wrong.
Explanation: Phenoxide ion is stabilized by resonance which is not possible in alkoxide ion.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(v) Both assertion and reason are correct statements but reason is not correct explanation of assertion.
Explanation: Due to intramolecular hydrogen bonding o-Nitrophenol does not form hydrogen bond with water
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(iv) Assertion is a wrong statement but reason is correct.
Explanation: Bromination of phenol cannot be carried out in presence of Lewis acid.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(ii) Assertion and reason both are wrong statements.
Explanation: Correct assertion: Boiling points of alcohol are higher than that of ethers of comparable molecular mass.
Correct reason: Alcohols can form intermolecular hydrogen bonding while ethers cannot.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(iv) Assertion is a wrong statement but reason is correct.
Explanation: 141 pm
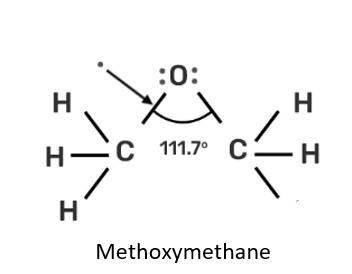
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(iv) Assertion is a wrong statement but reason is correct.
Explanation: Correct assertion is the IUPAC name of the compound is 1- (2-propoxy) propane.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(i) Assertion and reason both are correct and reason is correct explanation of assertion.
Explanation: P-nitrophenol is more acidic as nitro group helps in the stabilization of the phenoxide ion by dispersal of negative charge due to resonance.
New answer posted
6 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(ii) Assertion and reason both are wrong statements.
Correct assertion: Addition reaction of water to but-1-ene in acidic medium yields butan-2-o1.
Correct reason: Addition of water in acidic medium proceeds through the formation of secondary carbocation.
New answer posted
6 months agoContributor-Level 10
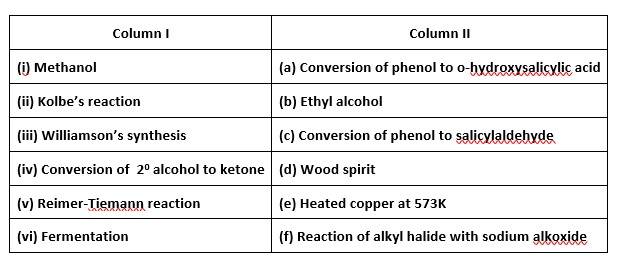
This is a matching answer type question as classified in NCERT Exemplar
(i)- (d); (ii)- (a); (iii)- (f); (iv)- (e); (v)- (c); (vi)- (b)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers