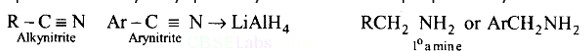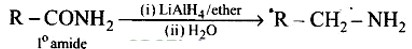Amines
Get insights from 162 questions on Amines, answered by students, alumni, and experts. You may also ask and answer any question you like about Amines
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option a and c
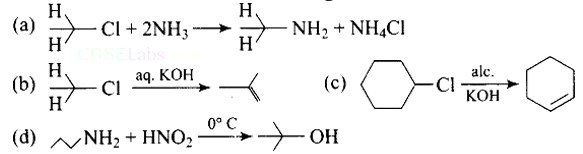
(a) Primary alkyl halides react with ammonia to give primary amines. Correct reaction.
(B) Elimination process doesn't occur with aq. KOH, hydrolysis process takes place. (B) is incorrect.
(c) Dehydrohalogenation I.e. elimination of HCl occurs which produces alkene as the product takes place with alc. KOH . (c) is correct.
(d) In this reaction, aliphatic primary amines, on treatment with nitrous acid, produce primary alcohol as a product.
Therefore (d) is incorrect.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (a and b)
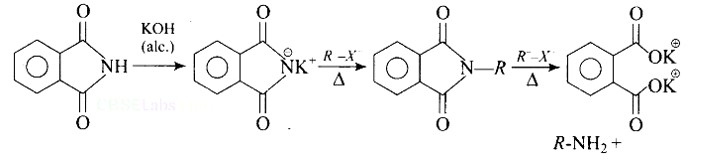
Gabriel synthesis is a method for producing primary amines. When phthalimide is treated with ethanolic potassium hydroxide, it forms a potassium salt of phthalimide, which when heated with an alkyl halide and then alkaline hydrolyzed yields the corresponding primary amine.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
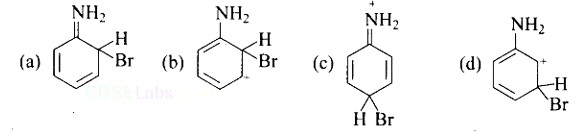
Ans: A, B & C
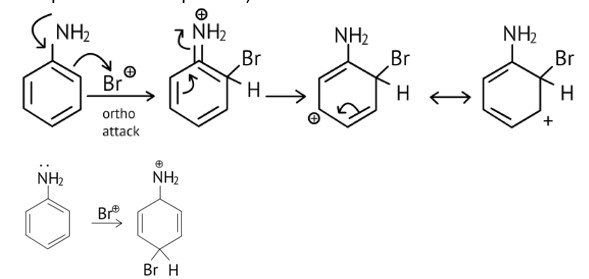
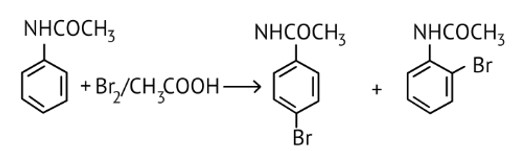
As the NH2 group is Ortho para directing group, so Br+ attacks only at the o and p positions thus the products formed corresponds to the option A, B and C.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
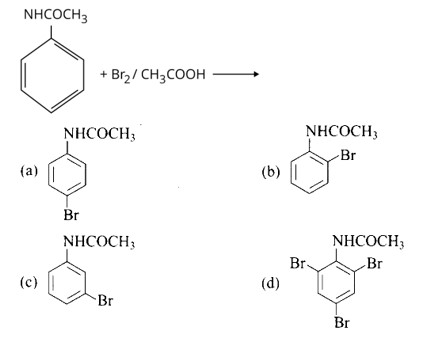
Ans: Option a and b
NHCOCH3 is an ortho- para directing group so ortho-para products are formed.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: option (B and C)
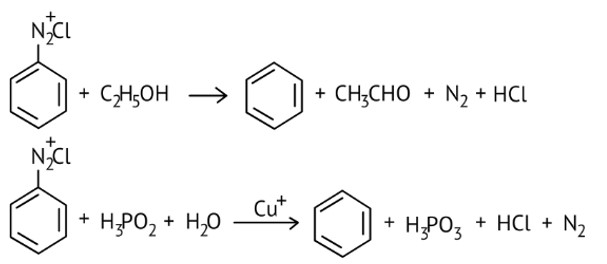
Certain mild reducing agents like hypophosphorous acid (phosphinic acid) or ethanol reduce diazonium salts to arenes and themselves get oxidised to phosphorous acid and ethanol, respectively.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (A and B)
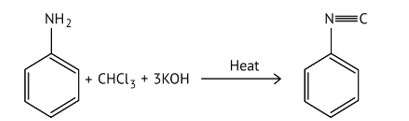
When aliphatic and aromatic primary amines are heated with chloroform and ethanolic potassium hydroxide, they form isocyanides or carbylamines, which have a foul odor. This reaction does not occur in secondary or tertiary amines.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (A, B & C)
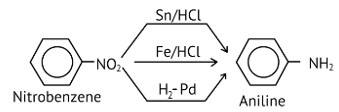
Nitro compounds are reduced to amines by passing hydrogen gas through an acidic medium containing finely divided nickel, palladium, or platinum, as well as by reduction with metals in an acidic medium.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (C and D)
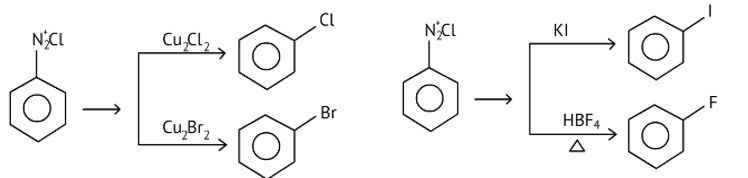
Chlorobenzene and bromobenzene are prepared by the sandmeyer's reaction.
While fluorobenzene and iodobenzene are prepared by simple heating of diazonium salt with aqueous KI Solution.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
Aliphatic and arylalkyl primary amines can be prepared by the reduction of the corresponding nitriles with LiAlH4
Heating alkyl halide with primary, secondary and tertiary amine can be prepared by reduction of LiAlH4 followed by treatment with water.
Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis produces primary amine. This process is known as Gabriel phthalimide reaction. The number of carbon atoms in the chain of amines of product is same as reactant.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
Among primary, secondary, and tertiary amines, tertiary amines are the most volatile compounds as they don't have any strong intermolecular H-bonding between N-H like the primary and secondary amines have.
So due to weak dipole-dipole interactions, (B) will be the most volatile.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 685k Reviews
- 1800k Answers