Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven
Get insights from 115 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
In BF3, due to n−pπ back bonding between the vacant p-orbital of boron and filled p-orbital of fluorine. This Π− pπ back bonding is absent in case of hydrogen as it is a single electron element.
Two BH3 molecules dimerise to form diborane.
In B2H6 There are two types of hydrogens present.
(I) Four hydrogens that are terminally bonded to each of two boron atoms.
(II) Two hydrogens that are bonded to both boron atoms forming a bridge in between.
The four terminal hydrogen atoms and two boron atoms lie in the same plane while bridging hydrogen lies in a plane perpend
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
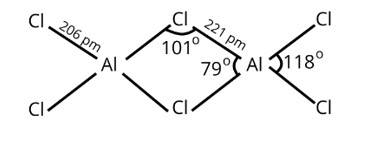
Both the compounds, BCl3 and AlCl3 are electron deficient compounds. In BCl3, boron is smaller in size and cannot assemble four big chlorine atoms near it causing steric hindrance and making it unstable.
Hence, BCl3 exists as a monomer only.
In AlCl3, aluminum has 3p-orbitals through which chlorine atoms can be accommodated easily to complete its octet and dimer is formed.
New answer posted
8 months agoContributor-Level 10
(i) TlCl is more stable than TlCl3, due to inert pair effect, +1 oxile. oxidation state.
(ii) AlCl3, Al3+ is more stable than aluminum ions in +1 state.
(iii) Due to the inert pair effect, +1 oxidation state is more stable than the +3 oxidation state. So, InCl is more stable than InCl3.
(iv) Boranes correspond to alkane-like compounds of boron. They consist of boron and hydrogen. Most common borane existing is dibecane.
4BF3 + 3LiAlH4?2B2H6 + 3LiF + 3AIF3
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
When aqueous solution of borax is acidified with hydrochloric acid, boric acid is produced. As the name says, boric acid is acidic in nature but weak acid. Unlike protonic acid, boric acid is monobasic acid. It accepts electrons from the hydroxyl group of water and forms [B (OH)4]-.
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
When aqueous solution of borax is acidified with hydrochloric acid, boric acid is produced. As the name says, boric acid is acidic in nature but weak acid. Unlike protonic acid, boric acid is monobasic acid. It accepts electrons from the hydroxyl group of water and forms [B (OH)4]-.
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
(i) ln? AlCl3, Aluminium is bonded to 3 chlorine atoms through three covalent bonds and six electrons are shared in the structure. To have complete Octet, AlCl3 lacks two electrons & hence act as an electron acceptor substance or Lewis acid.
(ii) Undoubtedly, fluorine has more electronegativity than chlorine, BF3 is stronger Lewis acid than BCl3 because of n−p π back bonding in BF3, both the constituting atoms boron and fluorine are involve p-orbital in back bonding. On moving down the group, the size of halogen atoms increases, back bonding decreases and Lewis ac
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
(i) Grp-13 The atomic size of the boron family follows the irregular trend. Generally, down the group the size increases but Gallium has a smaller atomic radius than Aluminium due to the poor shielding effect of 3d-orbitals.
Order: B
Grp-14 The size of the carbon family is smaller than the modern family and as we move down the group the atomic size increases regularly. The increase in covalent radius from carbon to silicon is prominent while from Silicon to lead a small increase in covalent radius is observed; this is due to the presence of completely filled D and f-o
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
Grp-13 The atomic size of the boron family follows the irregular trend. Generally, down the group the size increases but Gallium has a smaller atomic radius than Aluminium due to the poor shielding effect of 3d-orbitals.
Order: B
Grp-14 The size of the carbon family is smaller than the modern family and as we move down the group the atomic size increases regularly. The increase in covalent radius from carbon to silicon is prominent while from Silicon to lead a small increase in covalent radius is observed; this is due to the presence of completely filled D and f-orbita
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
