Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven
Get insights from 115 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
(B) & (D)
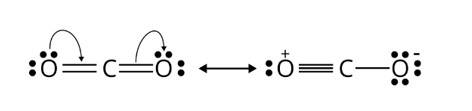
The following is the resonance structure of CO2.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
(A), (B) & (D)
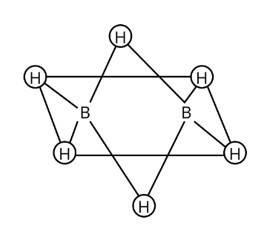
Two hydrogens forming bridge in B2H6 are peculiar in bonding and can be termed as 3 -centered-2-electron bond or banana bond. 1 s orbital of each hydrogen overlaps with the hybrid orbital of one of the boron then delocalising the 2e−over three atoms making 3 -centered-2-electron bond.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
(B) & (C)
(B) Fullerene is an allotrope of carbon consisting of 60 carbon atoms bonded together through single and double bonds in such a manner that they form a hollow sphere like shape or a cage-like structure.
(C) In graphite, each carbon atom has one free/ delocalised electron that causes strong attraction between carbon atoms providing more stability to the overall structure of graphite
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
(A) & (B)
Me3SiCl is a soleuless liquid and is stable in anhydrous condition. When added in the process of polymerization, it blocks the end terminal of the silicone polymer and determines its chain length.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
(B) & (C)
Hybridisation of any atom in a molecule corresponds to the number or σ bonds it is making covalently with other atoms.
In CO2, carbon is attached to two oxygen atoms through double bonds. Out of which two are σ bonds and the rest two are π bonds forming I−p π bonding between carbon and oxygen.
The two σ bonds correspond to the hybridization sp and give a linear shape to CO2.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
(A) & (B)
The atomic radii decrease as one moves down the group from Al to Ga due to the shielding effect of electrons. As a result of this ineffective effect, the effective nuclear charge rises.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
B
Cement is made by combining lime ( CaO ), clay with silica ( SiO), and oxides of aluminum, magnesium, and iron.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
C
Explanation:
Solid CO2 is referred to as dry ice because it is used in laboratories to create an ice bath for organic reactions. It is made by rapidly cooling high-pressure CO2 gas.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
D
Reducing agent are compound that donates electron to an electron recipient compound. Reducing agents oxidize themselves and reduce the other compounds. They easily lose electrons and increase their oxidation state; those electrons are accepted by electron needed compounds.
In SnCl2, Sn is in +2 oxidation state and can easily lose its two electrons and oxidize to +4 stable oxidation state.
New answer posted
8 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
B
Quartz is a crystalline form of silica that can be converted into other crystalline forms at high temperatures. It's commonly used as a piezoelectric material.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers