Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven
Get insights from 115 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
(A) Carbon has small size and large electronegativity, it forms strong n−pπ bonding with two oxygen atoms forming a separate CO2 molecule.
While in SiO2 silicon is larger in size with comparatively less electronegativity than carbon it shows no tendency to form n−pπ bonding rather forms Single covalent bond with oxygen. Thus, SiO2 possess 3D network-like structure in which each Silicon is bonded to 4 oxygen atoms.
(B) Carbon is smaller in size and lacks d-orbitals hence can have a maximum coordination number of four and sp3 hybridisation only.
Wherea
New answer posted
8 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
(A) sarbon in CCl4 does not have a vacant d-orbital to accommodate the electrons from OH of water molecules. Also CCl4 is nonpolar covalent compounds whereas H2O is polar. So, no strong interaction occurs between them. Hence CCl4 is miscible in water.
Whereas in SiCl4, silicon has bigger size than carbon and have d-orbitals for accommodation of electrons donated by OH of water in the process of hydroxylation. This leads to a strong interaction and silicon acid Is formed as a product. SiCl4 is completely miscible in water.
(B) As we move from carbon to sil
New answer posted
8 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
Both BCl3 and AlCl3 are electron deficient compounds that are central atom boron and aluminium have incomplete Octet. In each compound, a metal atom is surrounded by six electrons of three covalent bonds with 3 chlorine atoms.
Each chlorine atom has a complete Octet of eight electrons. The electron deficient compounds act as Lewis acid and readily accept two electrons to complete their octet.
New answer posted
8 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
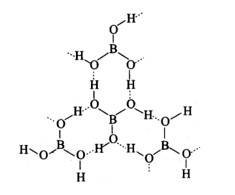
H3BO3
Boric acid forms a hexagon and rings through hydrogen bonding and has a layer-like structure.
Boric acid is present in water as [B (OH)4]−.H3BO3 electron from the OH of water and forms the complex BOH for negative for sp3 and is present in sp3 hybridisation.
Reaction:
B (OH)3+2H2O→ [B (OH)4]− + H3O+
New answer posted
8 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
H3BO3 or B (OH)3 is an electron deficient compound or Lewis acid which easily accepts electron from OH of water and releases its proton hence it is a Monobasic acid.
Reaction:
B (OH)3 + 2H2O → [B (OH)4]− + H3O+
New answer posted
8 months agoContributor-Level 10
This is a Short Answers Type Questions as classified in NCERT Exemplar
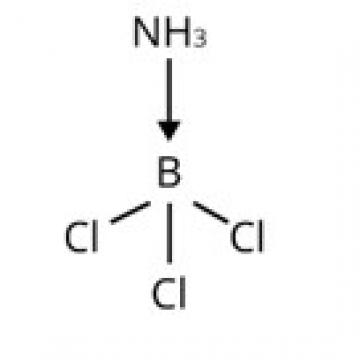
The central B atom in BCl3 has six electrons in its valence shell. As a result, it is an electron-deficient molecule in need of two more electrons to complete its octet. To put it another way, BCl3 acts as a Lewis acid. NH3 on the other hand, has a lone pair of electrons that it can easily donate. As a result, NH3 serves as a Lewis base. As shown below, the Lewis acid (BCl3) and Lewis base (NH3) combine to form an adduct:
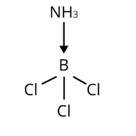
The valence shell of AlCl3 contains six electrons. As a result, it is an electron-deficient molecule that requires two additional electrons to compl
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
2C (s) + O2 + 4N2 (g) ![]() 2CO (g) + 4N2 ( g)
2CO (g) + 4N2 ( g)
Fe2O3 (S)+3CO (g) ![]() 2Fe (S)+3CO2
2Fe (S)+3CO2
C= tetravalent carbon
CO= carbon monoxide
Fe2O3= ferric oxide
CO2= carbon dioxide
Tetravalent elements i.e. carbon combines with oxygen to produce carbon monoxide. The reaction occurs at high temperatures and in the presence of nitrogen gas, which acts as a producer gas only. It is not consumed in the reaction. The carbon monoxide formed acts as a reducing agent for ferric oxide, reduces the oxidation state of iron from +3 to zero, and oxidiz
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
Boron is the only non-metallic and extremely hard element in group 13, and it is also used to make bulletproof vests. Boron exists in a variety of allotropic forms. It usually has a high melting point and no d orbital. Using 2s and 2p orbitals, it can achieve a maximum covalency of 4 . Because the octet of boron is not completed in trivalent halides of boron, it acts as Lewis acid. It forms an adduct when it reacts with Lewis base.
BF3+NH3→H3N−B−F3
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
B2H6 + 2NMe3→ 2BH3NMe3 BH3NMe3+H2O → H3BO3 + NMe3 + 6H2
(A) (B) (C)
(A): B2H6
(B): 2BH3NMe3
(C): H3BO3
New answer posted
8 months agoContributor-Level 10
This is a Long Answers Type Questions as classified in NCERT Exemplar
(i) Organosilicon polymers having (R2SiO2) as monomer units are called silicones. Silicones contain organic side groups which surround it giving alkane like nature and makes it hydrophobic. Silicones are applicative in electrical insulators, water proofing, sealant. They have been utilized in the biological field in cosmetic implants and other surgeries.
(ii) Boranes correspond to alkane-like compounds of boron. They consist of boron and hydrogen. Most common borane existing is dibecane.
4BF3 + 3LiAlH4?2B2H6 + 3LiF + 3AIF3
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers