Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
Molar mass of C7H5N3O6 = 12 * 7 + 1 * 5 + 14 * 3 + 16 * 6 = 84 + 42 + 96 = 227 g/mol
Number of moles = = 3 mol
New answer posted
3 months agoContributor-Level 9
Alitame is an artificial sweetener. It is a second generation of dipeptide sweetener. It is 200 times sweeter than sucrose.
New answer posted
3 months agoContributor-Level 10
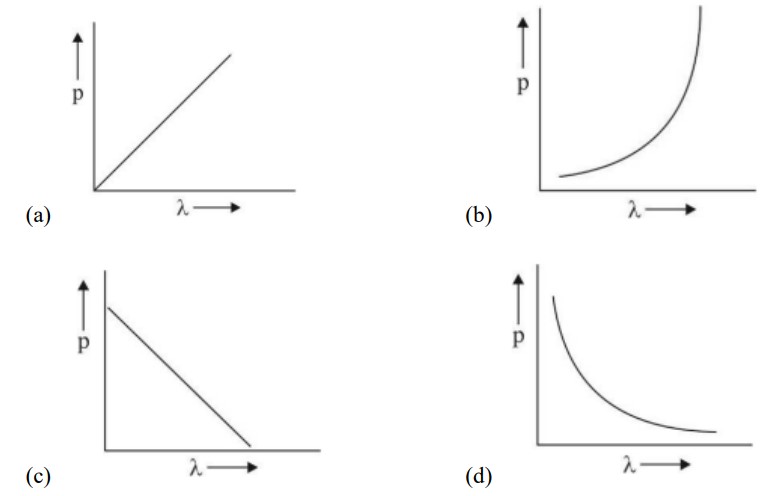
λ = h/p there is inverse relation between de-Broglie wavelength and momentum, hence the graph will be rectangular hyperbola.
New answer posted
3 months agoNew answer posted
3 months agoContributor-Level 10
(A) SiO? has three-dimensional network structure of Si – O bonds; while carbon dioxide consists of discrete CO? molecules. SiO? is solid, whereas CO? is a gas
(B) Because of its great affinity for oxygen, Si always occurs as the oxide, silica (SiO? ) or in the form of silicates, which are the compounds of SiO? with other metal oxides.
(C) SiO? + 2Mg → Si + 2MgO
(D) The reluctance of silicon to form pπ – pπ bonds to itself is clearly shown by the fact that silicon does not exist in graphite-like structure, but only in diamond like structure.
New answer posted
3 months agoContributor-Level 10
Kf = 1.86
Using, density of water = 1g / mL
i = ?
i = 1.344
Now, using
n for ClCH2COOH = 2
a =
Using
So; x = 36
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers