Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Nitrogen, sulphur and halogens are tested in an organic compound by lassaigne's test.
The organic compound is fused with sodium metal as to convert these elements into ionisable inorganic substances.
Na + C + N → NaCN
2Na + S → Na? S
2Na + X? → 2NaX
The cyanide, sulphide or halide ions can be confirmed in aqueous solution by usual test.
New answer posted
3 months agoContributor-Level 10
The process of moving sodium and potassium ions across the cell membrane is an active transport process involving the hydrolysis of ATP to provide the necessary energy.
New answer posted
3 months agoContributor-Level 10
The given value of µ (spin only)
2.84 = √n (n + 2) BM, So, n = 2
Among the given configurations, d? system in strong field ligand will have 2-unpaired e? in t? g set of orbitals as shown below.
New answer posted
3 months agoContributor-Level 9
Fe+2 undergoes reduction & I2 undergoes oxidation.
= (0.77 – 0.54) V = 0.23 V
= 23 * 10-2 V
x = 23
![]()
New answer posted
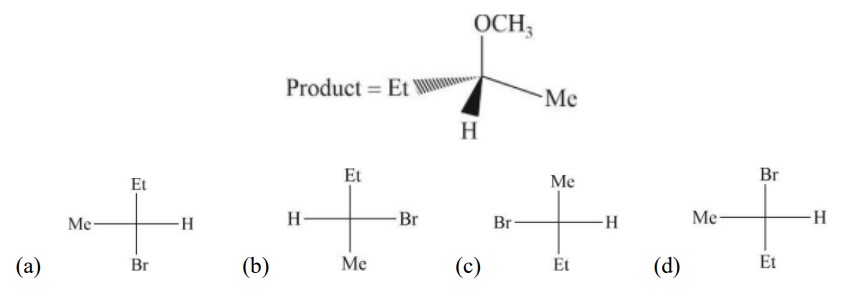
3 months agoContributor-Level 10
In S?2 reaction, inversion takes place, so configuration get reversed.
New answer posted
3 months agoContributor-Level 10
As Zn (OH)? , BeO, Al? O? are amphoteric, so they can react with both HCl and NaOH.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers