Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
Hydrated halides of group 2 from Ca onwards undergo dehydration on heating. But the hydrated halide of Mg, i.e., MgCl? ·8H? O, does not undergo dehydration on heating but undergoes hydrolysis.
BeO is amphoteric, while other oxides of group 2 are basic in nature.
New answer posted
3 months agoContributor-Level 9
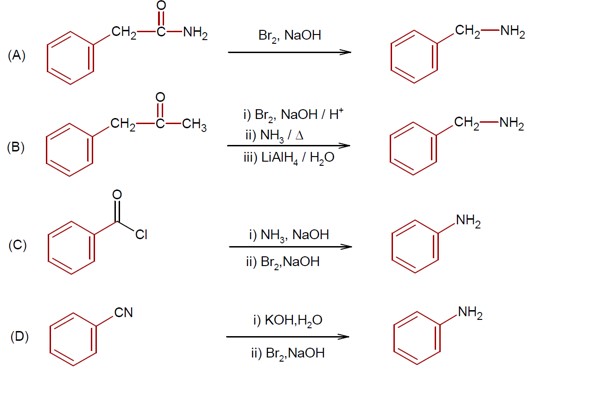
R-CONH? + Br? + 4NaOH → R-NH? + 2NaBr + Na? CO? + 2H? O
This reaction is the Hoffmann bromamide degradation, in which an amide is converted to a 1° amine.
New answer posted
3 months agoContributor-Level 9
Oxidation States (O.S) of Phosphorus (P):
- Hypophosphorous acid – H? PO? : +1
- Orthophosphoric acid – H? PO? : +5
- Hypophosphoric acid – H? P? O? : +4
- Orthophosphorous acid – H? PO? : +3
New answer posted
3 months agoContributor-Level 9
Formation of photochemical smog, which is also known as oxidizing smog, takes place in the presence of sunlight and O?
New answer posted
3 months agoContributor-Level 9
Purification of a compound is independent of the physical state of the pure compound in chromatography techniques.
New answer posted
3 months agoContributor-Level 9
H? O has a tetrahedral geometry and a bent shape with a bond angle of 104.5°, which is smaller than 109.5°. This is because of the presence of two lone pairs in H? O.
New answer posted
3 months agoContributor-Level 9
Fat-soluble vitamins are stored in our body for a relatively longer duration as compared to water-soluble vitamins.
- Vitamin B and C are water-soluble. (Thiamine is vitamin B1, while ascorbic acid is vitamin C).
- Vitamin A and vitamin D are fat-soluble, so they are stored in our body for a relatively longer duration.
New answer posted
3 months agoContributor-Level 9
The E° value for Ce? /Ce³? is +1.74 V, which suggests that Ce? is a strong oxidant, reverting to its common +3 oxidation state. So, Ce³? is more stable than Ce?
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers