Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
The Lassaign's test is a qualitative analysis method used to detect nitrogen, sulfur, phosphorus, and halogens in an organic compound. Copper (II) oxide is used to detect carbon. In the sodium fusion extract, halides (X? ) precipitate with AgNO? , and sulfide (S²? ) precipitates as black PbS.
New answer posted
3 months agoContributor-Level 9
Solubility of CdSO? is water ; S = 8 * 10? M
Using Ksp = S²
Ksp = 64 * 10? M
Now,
CdSO? Cd²? + SO? ²?
in H? SO? (0.01M)
Ksp = [Cd²? ] [SO? ²? ]
64 * 10? = S? (S? + 0.01)
S? << 0.01
So, S? = 64 * 10? M.
New answer posted
3 months agoContributor-Level 9
ΔG° = + 25.2 kJ / mol
Using ΔG° = -2.3 RT log Kp
25.2 * 10³ = - 2.3 * 8.3 * 400 log Kp
o 3.3 = log Kp
log (1 / 2*10³) = log Kp
Kp = 1 / (2*10³)
Using; Kp = Kc (RT)
1 / (2*10³) = Kc (0.083 * 400)? ¹
New answer posted
3 months agoContributor-Level 9
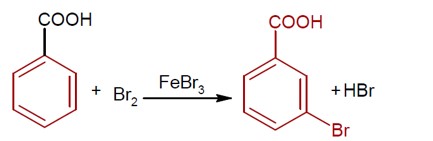
Moles of benzoic acid = 6.1 / 121 = 0.05
Theoretical moles of m- bromobenzoic acid = 0.05
Observed moles of m- bromobenzoic acid = 7.8 / 200 = 0.039
% yield = (0.039 / 0.05) * 100 = 78%
New answer posted
3 months agoContributor-Level 9
Half life, t? /? = 1min
Let, time of 99.9% completion of reaction be 't' min
Let the reaction is of first order
K = (2.303/t) log? ( [R]? / [R])
[R] = 0.001 [R]?
t = (2.303 * 3 min) / 0.693
t = 9.99 min
the nearest integer is 10.
New answer posted
3 months agoContributor-Level 9
λ° (BaCl? ) = 280 Scm²mol? ¹
λ° (H? SO? ) = 860 Scm²mol? ¹
λ° (HCl) = 426 Scm²mol? ¹
λ° (BaSO? ) = λ° (BaCl? ) + λ° (H? SO? ) - 2λ° (HCl)
= 280 + 860 - 2 * 426
= 288 Scm² mol? ¹
New answer posted
3 months agoContributor-Level 9
V (Na? CO? ) = 10 mL
Volume of HCl used will be 5 mL (average of titre values)
Meq of HCl = meq of Na? CO?
(M * n-factor * V)HCl = (M * n-factor * V)Na? CO?
0.2 * 1 * 5 = M * 2*10
M= 0.05 M
M= 50 mM
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers