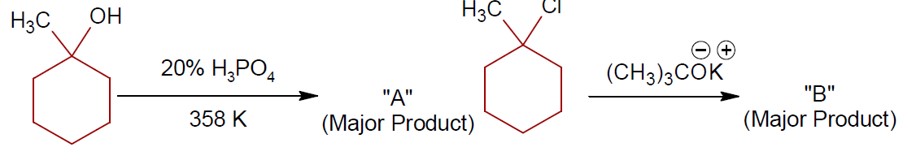
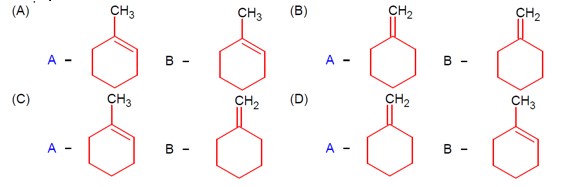
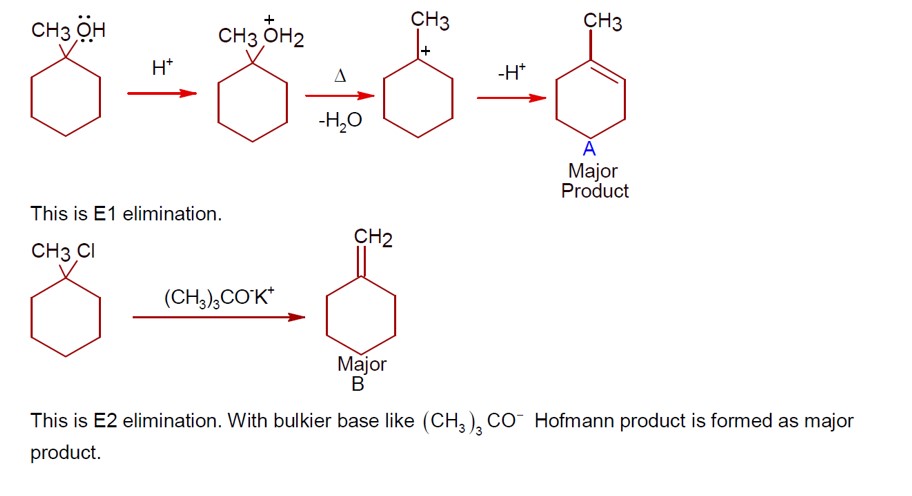
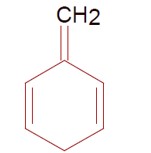
Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
The size of the Bk³? ion is less than the Np³? ion because Berkelium (Bk) lies beyond Neptunium (Np) in the actinoid series, and the size variation here is because of the actinoid contraction.
New answer posted
3 months agoContributor-Level 9
(a) Haber's process is used for the synthesis of NH?
(b) Ostwald's process is used to synthesize HNO?
(c) Contact process is used for the synthesis of H? SO?
(d) Hall-Héroult process is used for the extraction of aluminium.
New answer posted
3 months agoContributor-Level 9
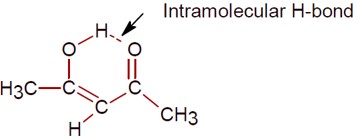
The enol form of acetone exists in less than 0.1% quantity, since its keto form is highly stable. But in the case of acetylacetone, the enol form is stabilized by intramolecular H-bonding, so its quantity increases to approximately 15%.
The intramolecular H-bond in the enol form of acetylacetone is shown.
New answer posted
3 months agoContributor-Level 9
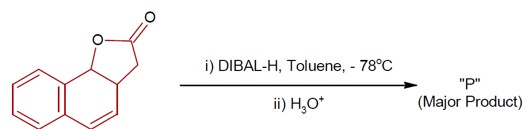
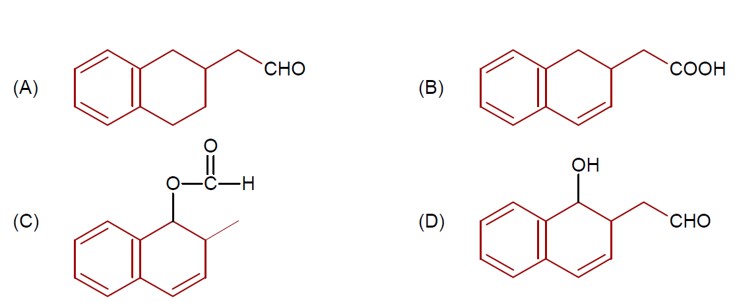
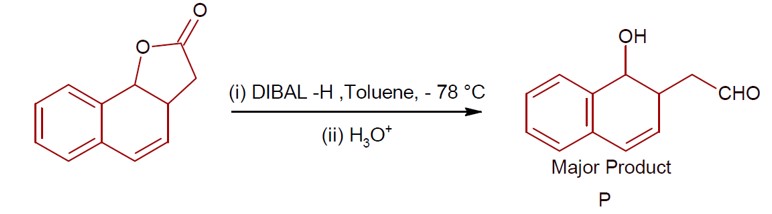
DIBAL-H at low temperature in a non-polar solvent, followed by hydrolysis, reduces esters to an aldehyde and an alcohol as a byproduct. The reaction shown is:
New answer posted
3 months agoContributor-Level 10
First, the specific conductance (κ) is calculated (κ = conductance * cell constant), and units are converted. Then, the molar conductivity (Λ? ) is found using the formula Λ? = κ / Concentration, with all units in the SI system. The calculation yields 14.3 mS m² mol? ¹.
Answer: 14 (Rounded off)
New answer posted
3 months agoContributor-Level 10
The energy is calculated using the formula Δ? = hc/λ. Plugging in the values for Planck's constant (h), the speed of light (c), and the given wavelength gives an energy of 3.99 * 10? ¹? J. The answer is requested as a single digit.
Answer: 4 (in units of 10? ¹? J)
New answer posted
3 months agoContributor-Level 10
Using Raoult's Law, P_Total = P°_A·X_A + P°_B·X_B = (21 kPa * 1/3) + (18 kPa * 2/3) = 7 + 12 = 19 kPa.
Answer: 19 kPa
New answer posted
3 months agoContributor-Level 10
The reaction is Fe (s) + 2HCl (aq) → FeCl? (aq) + H? (g). 50 g of Fe corresponds to 0.89 moles, which produces 0.89 moles of H? (g). The work done by the gas is calculated using W = -Δn_gasRT. The work done by the gas is the positive value, 2218 J.
Answer: 2218 J
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers