Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Ba has outer electronic configuration 6s2.
CaC2O4 is insoluble in water.
Compound of Li are covalent so soluble in organic solvent.
Na forms strong monoacidic base.
New answer posted
4 months agoContributor-Level 10
Sulphide ion (s2-) form ores commonly with Pb & Ag as PbS and Ag2S
New answer posted
4 months agoContributor-Level 10
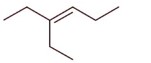
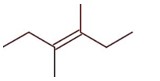
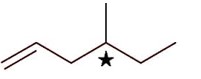
3-Ethylhex-3-ene will not show stereo isomerism
Methylhex-1-ene will show steroisomerism (optical isomerism)
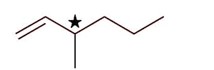
4-Methylhex-1-ene will show stereoisomerism (optical) since it has one chiral carbon
New answer posted
4 months agoContributor-Level 10
Diborane is produced when NaBH4 is reacted with I2
New answer posted
4 months agoContributor-Level 10
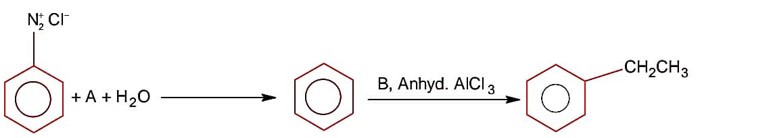
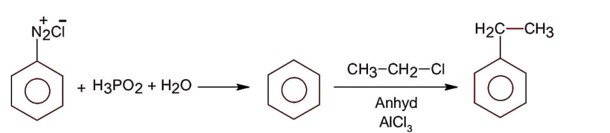
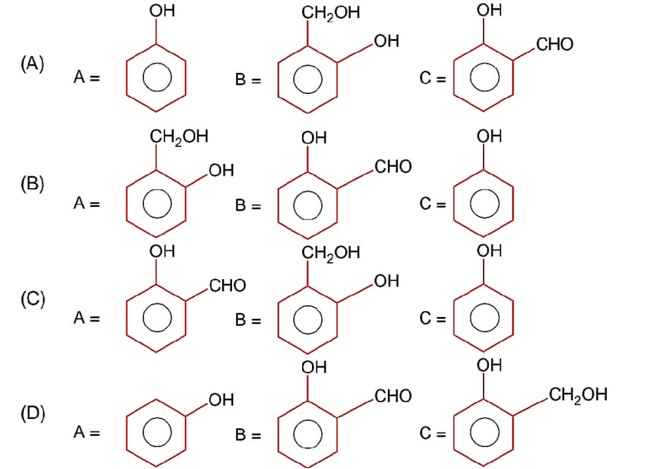
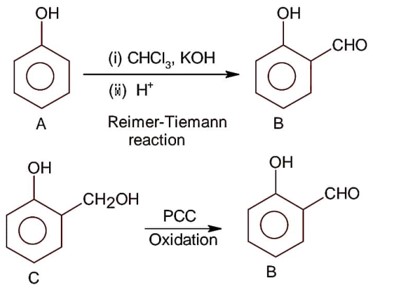
Compound A is phenol since phenol gives dark green colour with FeCl3.
New answer posted
4 months agoContributor-Level 10
(A) - Paramagnetic & coloured
- Diamagnetic & colourless
- Diamagnetic & colourless
(B) - Diamagnetic & colourless
- Diamagnetic & colourless
- paramagnetic & coloured
(C)
All are diamagnetic & colourless
(D)
All are paramagnetic & coloured
All d- & f block paramagnetic cations are coloured also.
New answer posted
4 months agoContributor-Level 10
Species | Sigma bonds | lone pairs | Hybridisation |
SF4 | 4 | 1 | Sp3d |
IF5 | 5 | 1 | Sp3d2 |
2 | 0 | Sp | |
4 | 0 | Sp3 |
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers