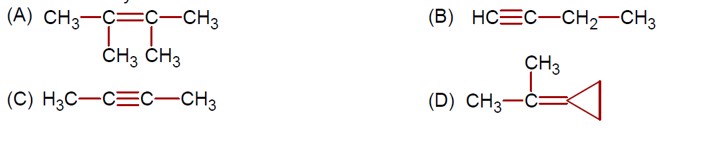Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
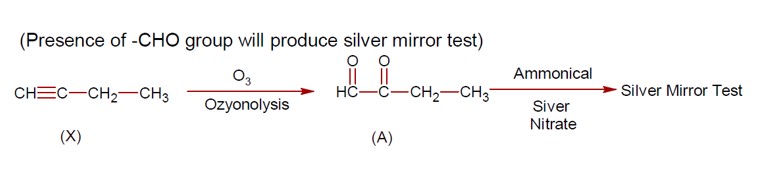
The Lassaign's test is a qualitative analysis method used to detect nitrogen, sulfur, phosphorus, and halogens in an organic compound. Copper (II) oxide is used to detect carbon. In the sodium fusion extract, halides (X? ) precipitate with AgNO? , and sulfide (S²? ) precipitates as black PbS.
New answer posted
4 months agoContributor-Level 9
ΔG° = + 25.2 kJ / mol
Using ΔG° = -2.3 RT log Kp
25.2 * 10³ = - 2.3 * 8.3 * 400 log Kp
o 3.3 = log Kp
log (1 / 2*10³) = log Kp
Kp = 1 / (2*10³)
Using; Kp = Kc (RT)
1 / (2*10³) = Kc (0.083 * 400)? ¹
New answer posted
4 months agoContributor-Level 9
V (Na? CO? ) = 10 mL
Volume of HCl used will be 5 mL (average of titre values)
Meq of HCl = meq of Na? CO?
(M * n-factor * V)HCl = (M * n-factor * V)Na? CO?
0.2 * 1 * 5 = M * 2*10
M= 0.05 M
M= 50 mM
New answer posted
4 months agoContributor-Level 10
The difference between the first and second ionization energies is significantly higher for alkali metals (like Sodium, Na) compared to alkaline earth metals (like Magnesium, Mg). Therefore, in the context of the problem, X=Na and Y=Mg.
New answer posted
4 months agoContributor-Level 10
Iron (III) iodide (FeI? ) does not exist because it is unstable. The Fe³? ion is a strong enough oxidizing agent to be easily reduced to Fe²? by the I? ion, which in turn is oxidized.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers