Class 12th
Get insights from 11.8k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
47. Option (ii) A (4) B (3) C (1) D (2)
Coloured bands are found in chromatography.
Hence, option (A) from column I is matched with option (4) from column II. Impure metals are converted to volatile complexes in Mond's process.
Hence, option (B) from column I is matched with option (3) from column II. Purification of Ge and silicon is done using zone refining.
Hence, option (C) from column I is matched with option (1) from column II. Purification of mercury is done using fractional distillation.
Hence, option (D) from column I is matched with option (2) from column II.
New answer posted
8 months agoContributor-Level 10
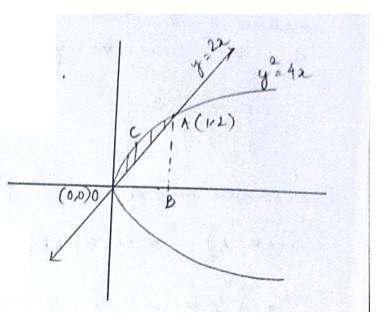
The given equation of the curve is - (1) and
the line is - (2)
Solving (1) and (2) for x and y
So,
for we get
for , we get
so, the point of intersection are (0,0)and (1,2)
area (DCAO)=area (DCABO)-area ( )
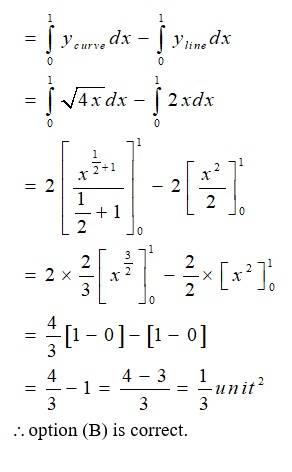
New answer posted
8 months agoContributor-Level 10
46. Option (ii) A (2) B (4) C (5) D (3)
Pendulum is always made of nickel steel.
Hence, option (A) from column I is matched with option (2) from column II. Malachite is the ore of copper.
Hence, option (B) from column I is matched with option (4) from column II. Calamine is the ore of zinc.
Hence, option (C) from column I is matched with option (5) from column II. Cryolite is an ore of aluminum.
Hence, option (D) from column I is matched with option (3) from column II.
New answer posted
8 months agoContributor-Level 10
28. Ionisation enthalpies are the main factor that influence the reactivity of transition elements. Higher the ionisation enthalpy, lesser is the reacting of the transition element.
When we move along the period from Sc to Cu, a regular increase in the ionisation enthalpy is observed which results in the almost regular decrease in the reactivity of elements.
New answer posted
8 months agoContributor-Level 10
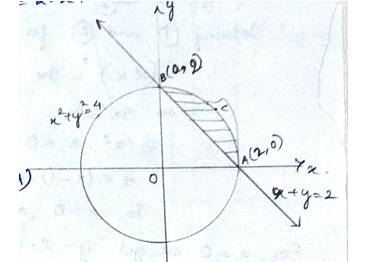
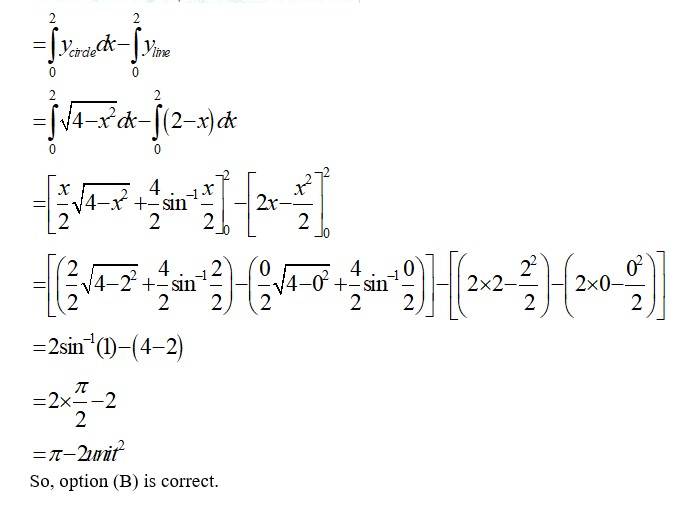
The equation of circle is which has centre at (0,0) & radius,
And the line
The smaller area of circle is given by
Area (ABCA) area (BOAB) – area (BOA)
New answer posted
8 months agoContributor-Level 10
27. As per (n + l) rule, 4s has lower energy than 3d-orbital.
3d−n+l = 3+2 = 5
4s - n + l = 4 + 0 = 4
So, 4s are filled first.
After filling of electrons, 4s-orbital moves beyond 3d-orbital and 4s electrons are loosely held by the nucleus. Hence, electrons are removed first during the process of ionisation.
New answer posted
8 months agoContributor-Level 10
The given equation of the sides of triangle is
--------------------(1)
-------------------(2)
-------------------------(3)
Solving eqn (1) and (2) for x & y we get
The point of inersection of line (1)and (2)is A (0,1)
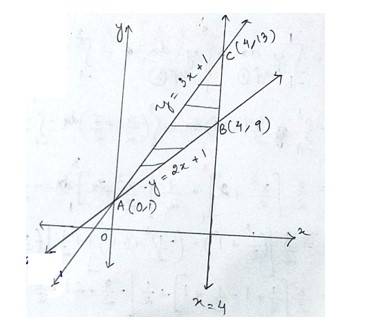
Putting x=4 in eq (1) and (2)we get,
The point of intersection of line (1)and (3) is B(4,9) and C (4,13)
Hence the required area enclosed ABC
New answer posted
8 months agoContributor-Level 10
45. Option (ii) and (iii)
Explanation: Using oxidation method for extraction of chlorine from brine. The reactions involved are:
2Cl− + 2H2O → 2OH− + H2 + Cl2
For this reaction, the value of ΔG°=+422 kJ, which is positive. Using the formula ΔG°=−nE°F, we get a negative value of E° =−2.2 V.
New answer posted
8 months agoContributor-Level 10
26. As the positive charge of the ion increases or we can say that oxidation state of a transition element increases, its size decreases and as per Fajan's rule, more the charge on the metal ion, more is its tendency to form covalent compounds because positively charged cation attracts the electron cloud strongly towards itself.
New answer posted
8 months agoContributor-Level 10
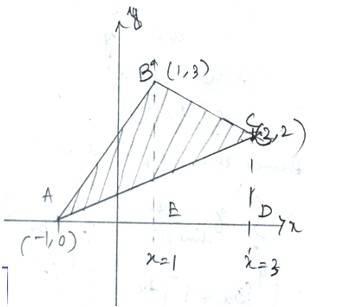
Let A (-1,0),B(1,3) and C (3,2) be the vertices of a triangle ABC
So, equation of line AB is
-------------(1)
Equation of line BC is
---------------(2)
Equation of line AC is
------------------------------(3)
Area of ABC= area ( )
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
