Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
Let 't' be the time taken by B to meat
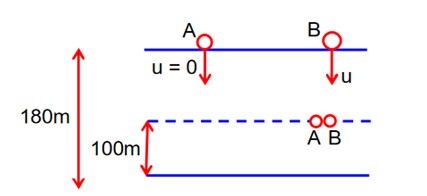
A.
for A, u = 0
t = [2 + t]
h = 80m
a = 10 m/sec2
h = ut +
80 = 0 * t +
16 = (2 + t)2
t + 2 = 4
t = 2sec
Now for B
h = ut +
80 = 2u + 5 * 2 * 2
80 = 2u + 20
2u = 60
u = 30m/sec
New answer posted
6 months agoContributor-Level 10
The structure consisting benzene ring is called benzenoidal structure and options (A) and (B) do not contain benzene ring, so they are non benzenoidal.
New answer posted
6 months agoContributor-Level 10
Match list-I with list-II
List-I Pollutant | List-II Source | ||
(a) | Microorganisms | (i) | Domestic sewage |
(b) | Plant nutrients | (ii) | Chemical fertilizer |
(c) | Toxic heavy metals | (iii) | Chemical factory |
(d) | Sediment | (iv) | Strip mining |
New answer posted
6 months agoContributor-Level 10
K2 [Cu (CN)4]
Oxidation number of Cu is +1
Cu+ = [Ar]3d10 ® Diamagnetic
New answer posted
6 months agoContributor-Level 10
Due to high lattice energy, LiF is sparingly soluble in water. Li+ has high hydration energy among its group members due to the smallest size.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
