Haloalkanes and Haloarenes
Get insights from 279 questions on Haloalkanes and Haloarenes, answered by students, alumni, and experts. You may also ask and answer any question you like about Haloalkanes and Haloarenes
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
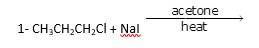
It is a simple substitution reaction with Cl being replaced by iodide ion.
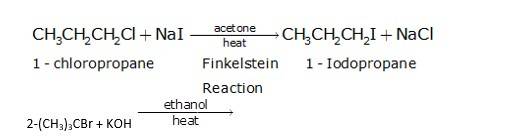
Since ethanol is a alcohol, so in presence of alcoholic KOH, alkyl chloride undergo elimination reaction, which results in the removal of proton and being substituted by a bromide ion.
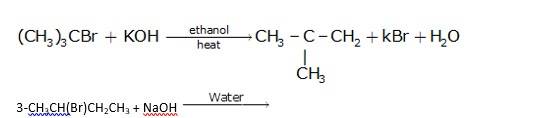
It is also a simple substitution reaction in which under the presence of aqueous NaOH, bromide ion is replaced by hydroxide ion as it is a better leaving group than hydroxide ion.
It is a simple substitution reaction in which a better leaving group leaves and is being substituted by an ion.
CH3CH2Br + KCN - CH3CH2CN + kBr
5-C6H5ONa + C2H5Cl
It is a simple substitution reaction in
New answer posted
8 months agoContributor-Level 10
(i) Freon 12:- the chlorofluorocarbon compounds of methane and ethane are collectively known as freons. They are extremely stable, unreactive, non-toxic and non-corrosive. Example of Freon 12 is CCl2F2. It is mainly used in refrigeration and eventually makes its way into the atmosphere where it diffuses unchanged into the stratosphere. In stratosphere, Freon is able to initiate the radical chain reactions that can upset the natural ozone balance.
- DDT: - it stands for p, p'-Dichlorodiphenyltrichloroethane (DDT). It is mainly used as an
- Iodoform: - it was earlier used as an antiseptic but the antiseptic properties are due the liberation o
New answer posted
8 months agoContributor-Level 10
Let us explain each question from Haloalkane and Haloarenes chapter one by one. Students who are currently in school and plan to take the CBSE board exam for class 12th soon, need to prepare all these questions. Let us get started.
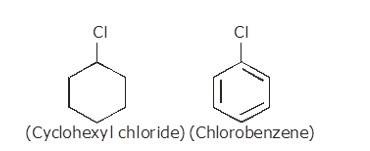
(i) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Sp2 hybrid carbon (s-character=33.33%) in chlorobenzene is more electronegative than a sp3-hybrid carbon (s-character=25%) in cyclohexylchloride, due to greater s-character. Thus, Carbon atom of chlorobenzene has less tendency to release electrons to Cl than carbon atom of cyclohexylchloride.
Chlorine atom in chlorobenzene is atta
New answer posted
8 months agoContributor-Level 10
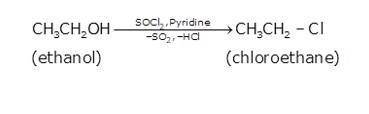
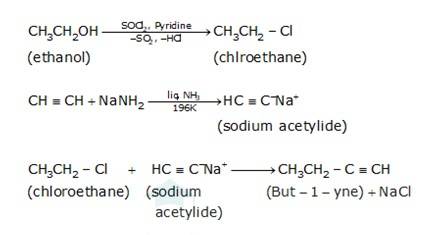
(i) Since the conversion of ethanol to but-1-yne involves the addition the two extra carbons that is why the conversion is carried out in 3 steps.
- In the first step ethanol is treated with thionyl chloride (SOCl2) in the presence of pyridine to give chloroethane
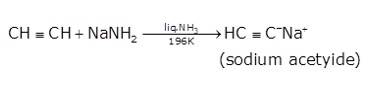
- In the second step the acetylene is treated with sodamide(NaNH2) in the presence of liquid ammonia to give sodium acetylide
- The last step involves the reaction between chloroethane and sodium acetylide to give the final product as the But-1-yne and NaCl as the by-product.
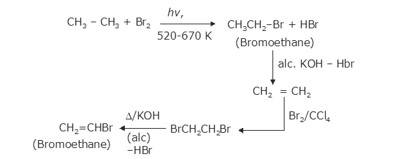
- The bromination of ethane (Br2 in the presence of light at 520-670K) gives bromoethane which on further treatme
New answer posted
8 months agoContributor-Level 10
Carbon due to its chemical property of catenation, tetravalency and the ability to form stable covalent bonds form hydrocarbon and halo-alkanes easily.
The process of removal of hydrogen and halogen from adjacent carbon atoms of haloalkens is called dehydrohalogenation. NaOEt ( Sodium ethoxide) used in Dehydrohalogenation. It is a strong base and helps remove a β-hydrogen from the haloalkane.
The chemical reaction for
Reaction:
R–CH2 –CHX–R′+NaOEt ethanol > R–CH=CHR'+NaX+EtOH
(i) 1-Bromo-1-methylcyclohexane:
Br
|
CH3–C1–cyclohexane&
New answer posted
8 months agoContributor-Level 10
(i) Since I- ion is a better leaving group than Br- ion, therefore, CH3I reacts faster CH3Br in SN2 reaction with OH- ion.
Better the leaving group, faster is the SN2 reaction.
(ii) On steric grounds, 1? alkyl halides are more reactive than tert-alkyl halides in SN2 reactions. Therefore, CH3Cl will react at a faster rate than (CH3)3CCl in a SN2 reaction with OH- ion. (Bulkier the group, slower is the SN2 )
New answer posted
8 months agoContributor-Level 10
Ambident Nucleophiles are those nucleophilies which can attack through different sites. For example:-cyanide ions are a resonance hybrid of the following two structures:

It can attack through carbon to form cyanide and through N to form is O cyanide. Example: NO2, NO3 - etc.
New answer posted
8 months agoContributor-Level 10
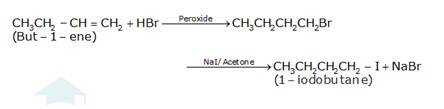
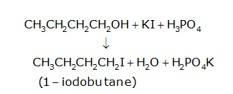
(a) 1-butanol is treated with KI in the presence of H3PO4 where the OH is being replaced by the iodine and gives H2O and KH2PO4 as the by-product with 1-iodobutane as the final
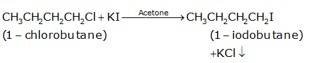
(b) The conversion of 1-chlorobutane to 1-iodobutane simply takes place by treating the reactant with KI in the presence of acetone. The iodine from KI normally replaces the chlorine from the reactant and gives 1-iodobutane as the final product with KCl as the by product.
(c) The conversion of but-1-ene to 1-iodobutane takes place according to anti-markovnikoff (positive charged ion H+ goes to the carbon which has less number of hydrogens and negative part Br-
New answer posted
8 months agoContributor-Level 10
Double bond equivalent is used to find the level of unsaturations present in an organic molecule.
DBE = C +1-H/2+X/2-N/2
Where C= number of carbon atoms present H=number of hydrogen atoms present N=number of nitrogen atoms present X=number of halogen atoms present Double bond equivalent (DBE) for C4H9Br
= 4+1-9/2+1/2
=0
So none of the isomers has a ring or unsaturation, so the isomers are positions or chain isomers as shown below in the table:

New answer posted
8 months agoContributor-Level 10
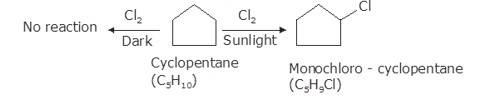
As per the molecular formula , one thing can be confirmed; the hydrogen is either cycloalkane or it is an alkene. However, alkenes readily react with chlorine in even dark because of the double bond. Therefore, it is possible that the hydrocarbon is cycloalkane.
Now, let us consider the reactivity. Cycloalkanes are the saturated hydrocarbons with no double bonds. These do not react with Chlore in dark because such a reaction needs UV light for initiating the process of free radical substitution. Haloalkane and Haloarenes NCERT solutions cover this as well as other questions in further detail.
In bright sunlight, the UV light star
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers