Haloalkanes and Haloarenes
Get insights from 279 questions on Haloalkanes and Haloarenes, answered by students, alumni, and experts. You may also ask and answer any question you like about Haloalkanes and Haloarenes
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
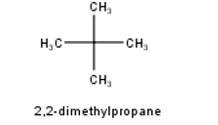
To have a single monochloride, there should be only one type of H-atom in the isomer of the alkane of the molecular formula C5H12. This is because of the fact that replacement of any H-atom leads to the formation of the same product. Therefore the isomer is 2,2-dimethylpropane.
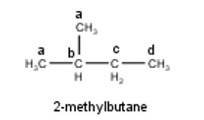
To have three isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12 should contain three different types of H-atoms. Therefore, the isomer is n-pentane. It can be observed that there are three types of H atoms labelled as a, b and c in n-pentane as shown below : -
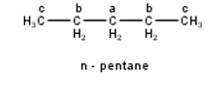
To have four isomeric monochlorides, the isomer of the alkane of the molecu
New answer posted
8 months agoContributor-Level 10
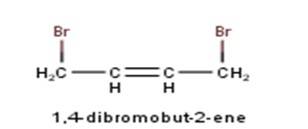
10.3 There are four different dihalogen derivatives of propane. The structures of these derivatives are as shown below: -
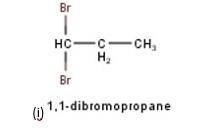

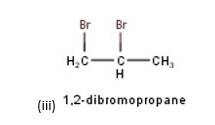
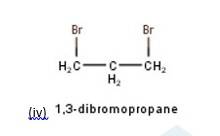
New answer posted
8 months agoContributor-Level 10
In the presence of sulphuric acid (H2SO4), KI produces HI as follows : -
2KI + H2SO4 → 2KHSO4 + 2KI
Since H2SO4 is an oxidising agent, it oxidises HI (produced in the reaction to I2)
2HI + H2SO4 → I2 + SO2 +;
As a result, the reaction between an alcohol and HI to produce alkyl iodide cannot occur. Therefore, sulphuric acid is not used during the reaction of alcohols with KI. Instead, a non-oxidising acid such as H3PO4 is used in the reaction to get the desired product.
A few things you can remember here, while solving NCERT Solutions for Haloalkanes And Haloarenes.
Sulphuric acid is a powerful oxidising agent. We know this because the su
New answer posted
8 months agoContributor-Level 10
10.1 From the name of the compound it is clear that the parent ring is pentane and chloro and methyl groups are attached in the straight chain at 2nd and 5th position respectively. Hence, the structure is as follows:
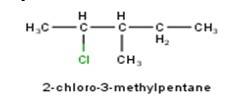
From the name of the compound given it is clear that the parent group is hexane with 2 attachments namely chloro and ethyl groups at 1 and 4 positions respectively. Hence, the structure is as follows: -
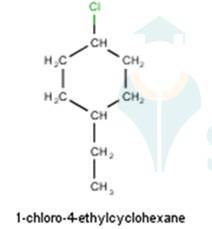
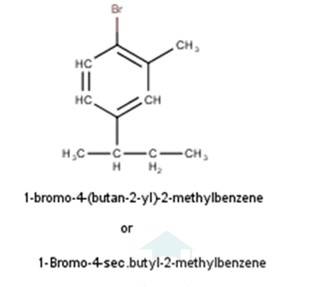
From the name of the compound given it is clear that the parent group is heptane with tertiary butyl and iodine groups attached at 4 and 3 positions respectively. Hence, the structure is as follows: -
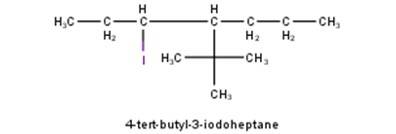
From the name of the comp
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers