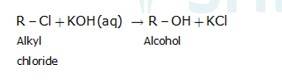Haloalkanes and Haloarenes
Get insights from 279 questions on Haloalkanes and Haloarenes, answered by students, alumni, and experts. You may also ask and answer any question you like about Haloalkanes and Haloarenes
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
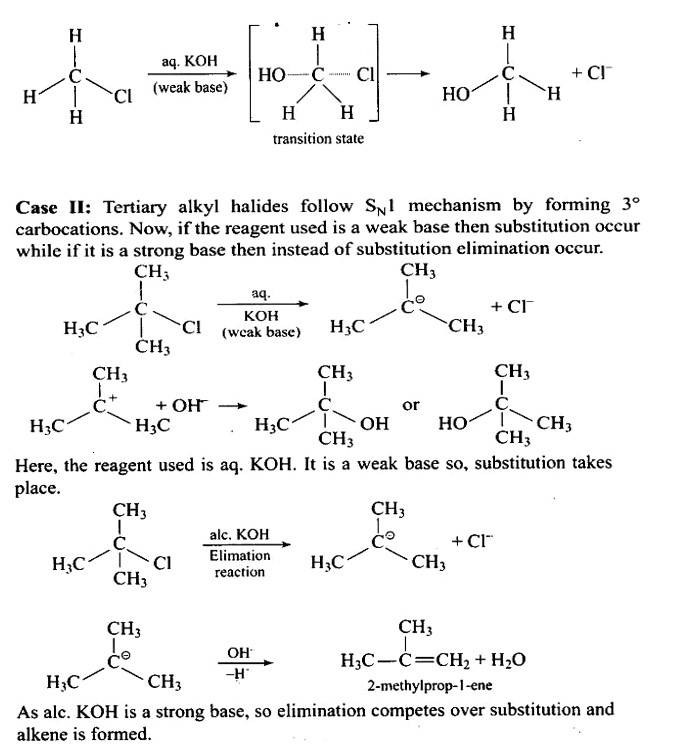
The structure of the alkyl halide as well as the chemicals used in the reaction dictate whether it undergoes substitution or elimination. The reactivity of alkyl halides to substitution processes can be examined to better understand their structural characteristics. The SN2 mechanism, which involves cleavage of the halide from the carbon atom and simultaneous attachment of the attacking nucleophile, is preferred by primary alkyl halides for substitution reactions.
Tertiary halides, on the other hand, go through an elimination process due to the production of stable carbo
New answer posted
8 months agoContributor-Level 10
This is a classic question from the chapter Haloalkanes and Haloarenes. Let us solve each one as follows:
(1) When n - butyl chloride is treated with alcoholic KOH, the formation of but - l - ene takes place. This reaction is a dehydrohalogenation reaction.
When N-butyl chloride or 1-chlorobutane is treated with alcoholic potassium hydroxide (KOH), elimination reaction takes place. This leads to alkene formation. 1-butene is the major product here.
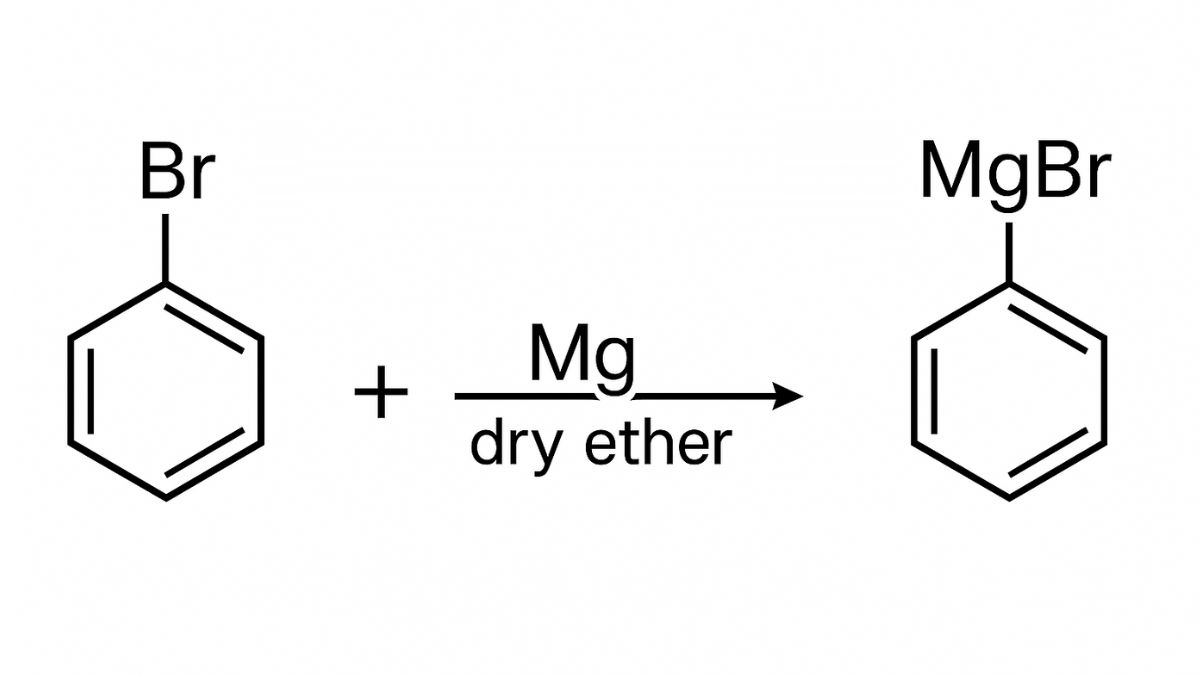
(ii) When Bromobenzene is treated with Mg in the presence of dry ether, it undergoes a reaction to produce phenylmagnesium bromide which is the Grignard reagent. This reagent is very re
New answer posted
8 months agoContributor-Level 10
There are two primary alkyl halides having the formulaC4H9Br, They are n - butyl bromide and isobutyl bromide.
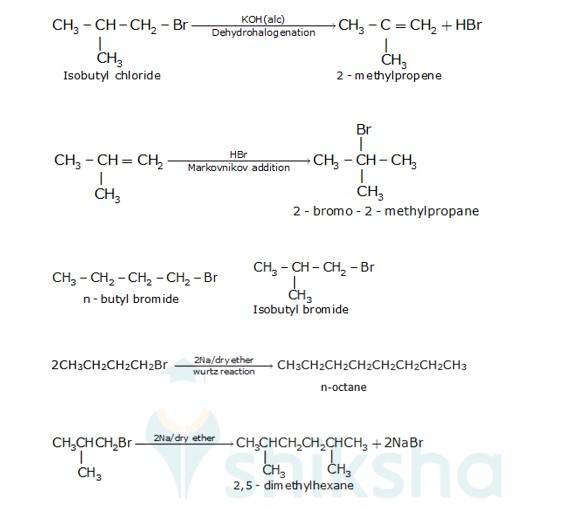
Therefore, compound (a) is either n-butyl bromide or isobutyl bromide.
Now, compound (a) reacts with Na metal to give compound (b) of molecular formula, C18H18 which is different from the compound formed when n-butyl bromide reacts with Na metal.
Hence, compound (a) must be isobutyl bromide. Thus, compound (d) is 2, 5-dimethylhexane.
It is given that compound (a) reacts with alcoholic KOH to give compound (b). Hence, compound (b) is 2- methylpropene.
Also, compound (b) reacts with HBr to give compound (c) which is an isomer of (a)
New answer posted
8 months agoContributor-Level 10
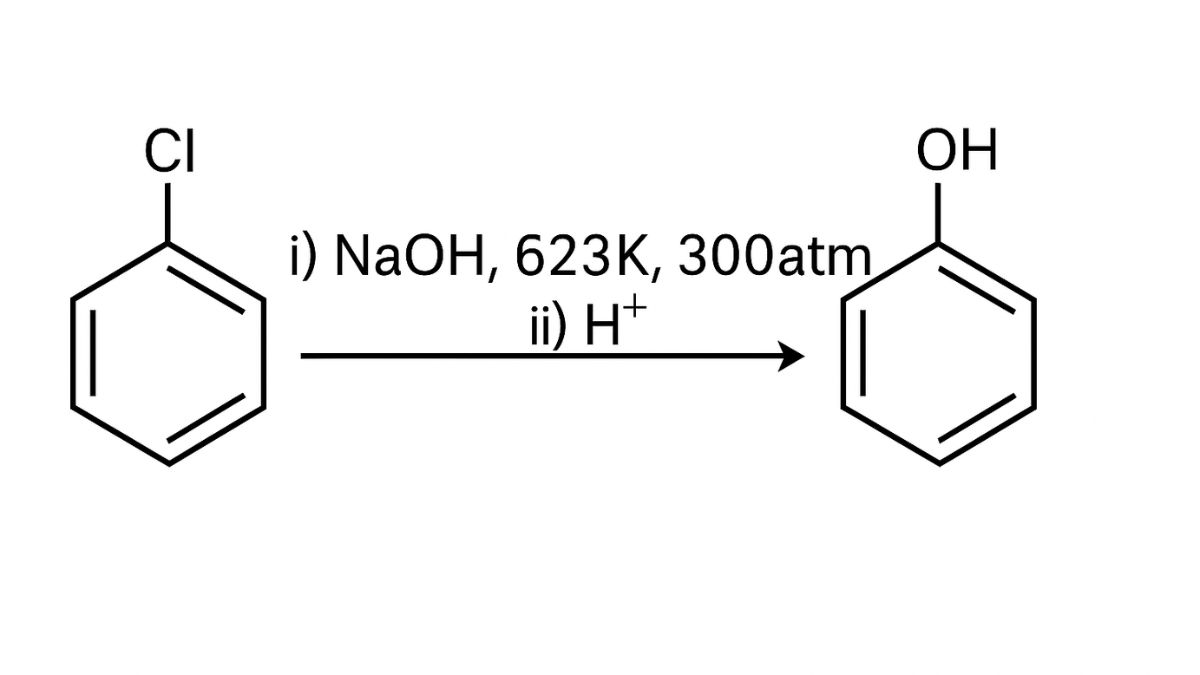
An aqueous solution, KOH almost completely ionizes to give OH-ions. OH- ion is a strong nucleophile (see the imp. note below), which leads the alkyl chloride to undergo a nucleophilic substitution reaction to form alcohol.
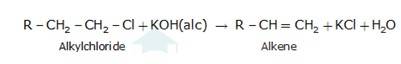
On the other hand, an alcoholic solution of KOH contains alkoxide (RO-) ion, it is a strong base. Thus, it can abstract a hydrogen ion from the beta-carbon of the alkyl chloride and form an alkene by eliminating a molecule of HCl.OH- ion is a much weaker base than RO- ion. Also, OH- ion is highly solvated (because more energy is released on solvation)in an aqueous solution and as a result, the basic character of OH-io
New answer posted
8 months agoContributor-Level 10
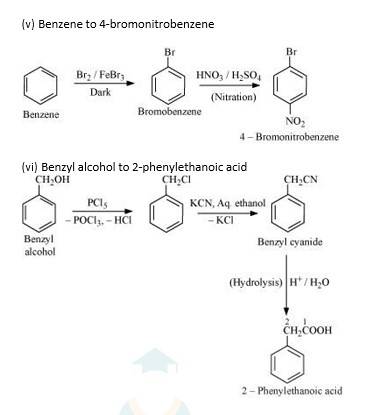
(i) Propene to propan-1-ol
It is an antimarkovnikoff reaction in which under the presence of a peroxide an alkene undergoes substitution, wherein the halo group is attached to that carbon which has least no. Of alkyl groups attached to it.
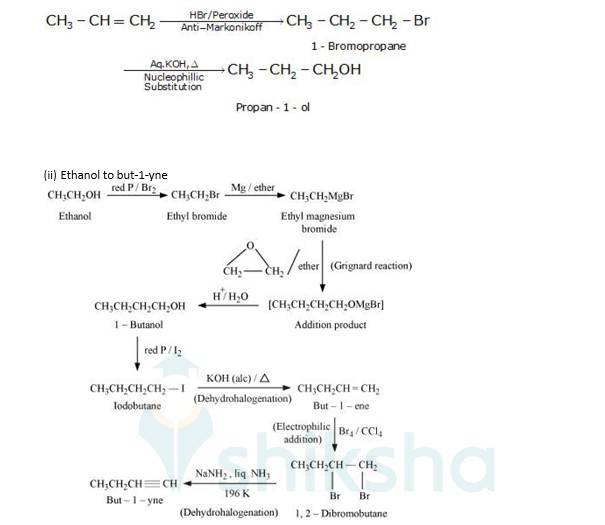
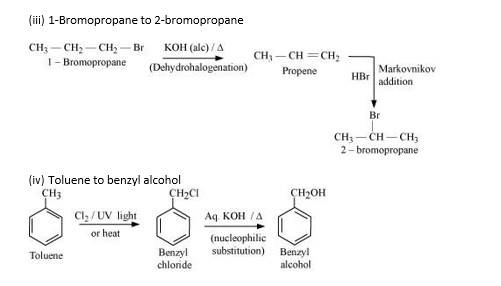
New answer posted
8 months agoContributor-Level 10
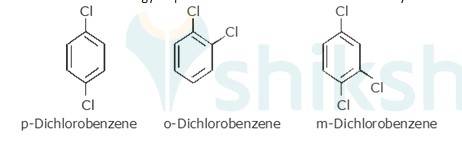
p-Dichlorobenzene is more symmetrical than o-and m-isomers. Because it fits more closely and easily in the crystal lattice than o-and m-isomers. Therefore, more energy is required to break the crystal lattice of p-dichlorobenzene. Therefore, p-dichlorobenzene is symmetrical and has a higher melting point and lower solubility than o-and m-isomers due to strong force of attraction in crystal.
Therefore more energy required to break lattice and will not easily be soluble.
New answer posted
8 months agoContributor-Level 10
Hydrolysis by KOH results in the formation of the carbocation. Those compounds which leads to the formation of stable carbocation are easily hydrolysed.C6H5CH2Cl leads to formation of 1°- carbocation, while C6H5CHClC6H5 forms 2°-carbocation, which is more stable than 1°-carbocation. Hence C6H5CHClC6H5, is hydrolyzed more easily than C6H5CH2Cl by aqueous KOH.
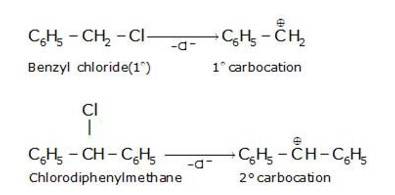
New answer posted
8 months agoContributor-Level 10
The reaction involves the approaching of the nucleophile to the carbon atom to which the leaving group is attached. When the nucleophile is sterically hindered (does not have any free space or very crowded), then the reactivity towards SN2 displacement decreases. Due to the presence of substituents, hindrance to the approaching nucleophile increases in the following order.
- Bromopentane < 2-bromopentane < 2-Bromo-2-methylbutane. The structures are shown below:
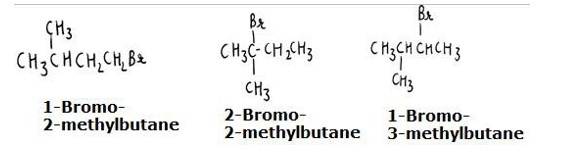
Hence, the increasing order of reactivity towards SN2 displacement is:
- Bromo-2-methylbutane < 2-Bromopentane < 1-Bromopentane
- The stearic hinderance in alkyl halides increases in the order of 1° < 2 < 3, the increasing order of reactivity towards SN2 displacement is given as-
3° < 2 < 1.
The structures are given below:

Hence, the given set of compounds can be arranged in the in
New answer posted
8 months agoContributor-Level 10
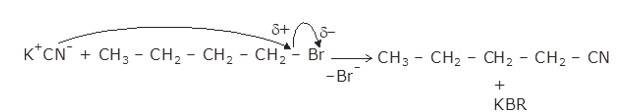
The given reaction is a nucleophillic reaction:

The given reaction is an SN2 reaction. In this reaction, CN acts as the stronger nucleophile and attacks the carbon atom to which Br is attached in nBuBr.
And, CN- ion is an ambident nucleophile and can attack through both C and N. In this case, it attacks through the C-atom.
The mechanism is shown below:
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers