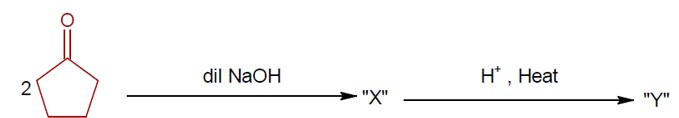
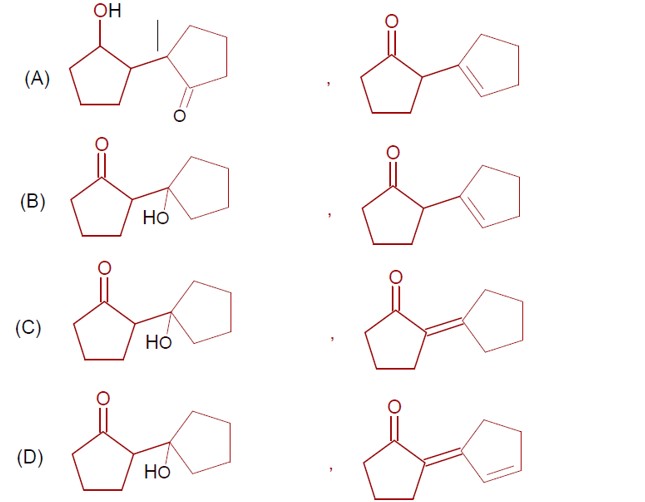
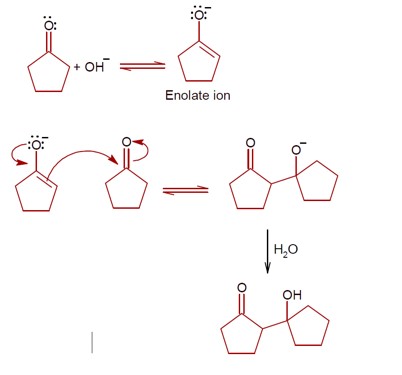
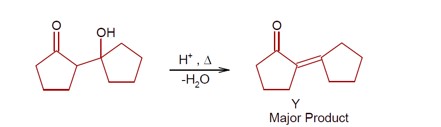
Haloalkanes and Haloarenes
Get insights from 279 questions on Haloalkanes and Haloarenes, answered by students, alumni, and experts. You may also ask and answer any question you like about Haloalkanes and Haloarenes
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
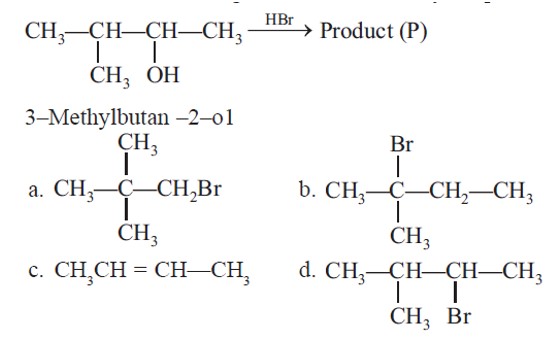
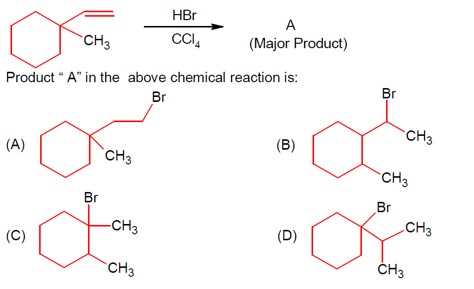
Initially, after protonation followed by loss of water, secondary carbocation is formed, further Hydride shift leads to 3° carbocation.
New answer posted
4 months agoContributor-Level 10
Element % At. Weight (% / At.weight) the simplest ratio
C 78 12 6.5 1
H 22 1 22 ≈ 3
New answer posted
4 months agoContributor-Level 10
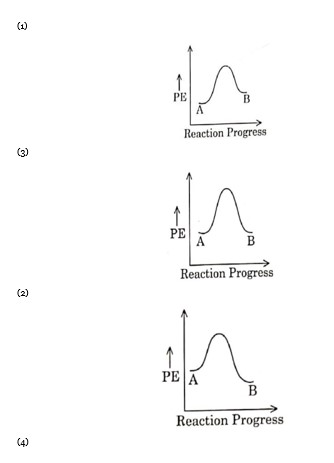
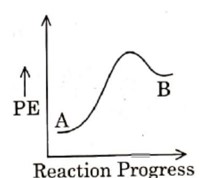
For a given reaction? H is negative. Hence, potential energy profile is of an exothermic reaction.
New answer posted
4 months agoContributor-Level 9
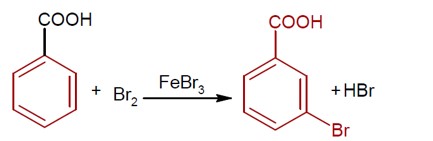
Moles of benzoic acid = 6.1 / 121 = 0.05
Theoretical moles of m- bromobenzoic acid = 0.05
Observed moles of m- bromobenzoic acid = 7.8 / 200 = 0.039
% yield = (0.039 / 0.05) * 100 = 78%
New answer posted
4 months agoContributor-Level 9
Both C? H? OH and AgCN can generate nucleophile.
KCN generate nitrile as nucleophile while AgCN generate isonitrile as nucleophile in nucleophilic substitution reaction.
New answer posted
4 months agoContributor-Level 9
o Let the number of atoms of element A be N.
o Tetrahedral voids = 2N.
o Atoms of element M = (2/3) * 2N = 4/3 N.
o The ratio M : A is (4/3 N) : N, which simplifies to 4:3.
o So, the formula of the compound is M? A?
New answer posted
4 months agoContributor-Level 10
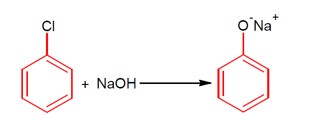
This describes a nucleophilic aromatic substitution reaction. Aryl halides are very less reactive toward this reaction, so the reaction takes place at a high temperature, i.e., 623K, and high pressure of 300 atm.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers