Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
5.35
We can say that colloid is not a substance but a state of a substance which is dependent on the size of particle colloidal state is intermediate between a true solution and a suspension.
When a size of substance is between 1nm to1000nm it behaves as colloid otherwise not.
New answer posted
6 months agoContributor-Level 10
1 - Methylethanamine
The root name is based on the longest chain with the -NH2 attached. The chain is numbered so as to give the amine unit the lowest possible number. The longest chain is ethane chain which is further suffixed with 'amine'.
2 - Propan-1-amine
The longest chain here is propane. The naming is such that amine unit should get a the lowest possible number. Propane-1-amine can also be written as 1-propylamine.
3 - N−Methyl-2-methylethanamine
The chain is numbered so as to give the amine unit the lowest possible number. The other alkyl group is treated as a substituent, with N as the locant. The N locant is listed before numeri
New answer posted
6 months agoContributor-Level 10
8.13 The oxidation states displayed by the first half of the first row of transition metals are given in the table below.
METALS | Sc ( [Ar] 3d14s2) | Ti ( [Ar] 3d24s2) | V ( [Ar] 3d34s2 ) | Cr ( [Ar] 3d54s1) | Mn ( [Ar] 3d54s2) |
OXIDATION STATES |
| +2 | +2 | +2 | +2 |
+3 | +3 | +3 | +3 | +3 | |
| +4 | +4 | +4 | +4 | |
|
| +5 | +5 | +6 | |
|
|
| +6 | +7 |
Except for Sc, all others metals display +2 oxidation state. This is because as the atomic number increases, the number of electrons in the valence shell increases. +2 oxidation state is attained by the loss of the two 4s electrons by these metals. As the number of electron increases, the possibility of an ion with +2 oxidation state being stable (by attaining half-filled structure) also increases. Finally, Mn2+ ions have half-filled structure and are very stable.
New answer posted
6 months agoContributor-Level 10
5.34
Alcohol- a colloidal solution having alcohol as the dispersion medium and a solid substance as the dispersed Ex- colloidal sol of cellulose nitrate in ethyl alcohol.
Aerosol- a colloidal solution having gas as the dispersion medium and a solid substance as the dispersed Ex-Smoke.
Hydrosol- a colloidal solution having Water as the dispersion medium and a solid substance as the dispersed Ex-Gold sol.
New answer posted
6 months agoContributor-Level 10
1- When 3-methylaniline treated with NaNO2 + HCl it gets converted into chlorine complex.
When that complex reacted with HBF4 It gets converted into Barium Fluoride complex. This complex reacts with NaNO2 in presence of copper to give 3-Nitrotoluene.
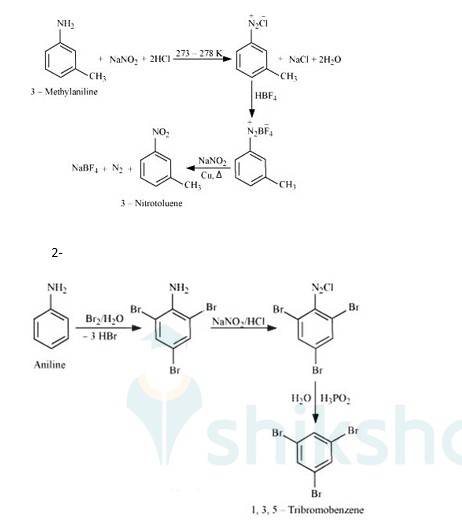
When aniline reacts with Br2 water it gets converted into 2,4,6 tribromobenzamine. When this further reacted with NaNO2/HCl it forms Chloride complex. This complex forms 1,3,5 tribromobenzene after treating with H3PO2 in presence of water.
New answer posted
6 months agoContributor-Level 10
8.12 Electronic configuration of Mn2+ is [Ar]183d5 and Electronic configuration of Fe2+ is [Ar]18 3d6 . It is known that half-filled and fully-filled orbitals are more stable. Therefore, Mn in (+2) state has a half-filled stable configuration, whereas the Fe in +3 oxidation state has partially filled subshells, which are relatively unstable. This is the reason Mn2+ shows resistance to oxidation to Mn3+. Also, Fe2+ has 3d6 configuration and by losing one electron, it attains half- filled stable Hence, Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state.
Mn+2→
Manganese has the atomic no. 25 and its electronic config
New answer posted
6 months agoContributor-Level 10
The different isomers of the molecular formula: C3H9N are given in the table. However only 1° amines will liberate nitrogen gas on the treatment with h=the nitrous acid are given as follows:
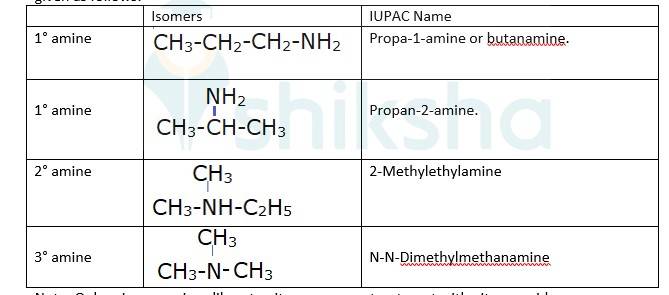
Note: Only primary amines liberate nitrogen gas on treatment with nitrous acid.
New answer posted
6 months agoContributor-Level 10
5.33
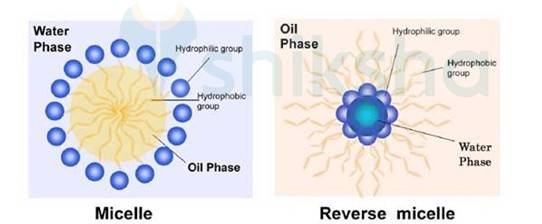
Soaps contain hydrophobic and hydrophilic part when dissolved in the water they arrange themselves in such a way that they form a spherical structure having hydrophobic part towards the centre and hydrophilic part away from centre. This cluster is known as Micelle. Ex-Sodium stearate + Water
(CH3 (CH2)16COO-Na + H2O)
New answer posted
6 months agoContributor-Level 10
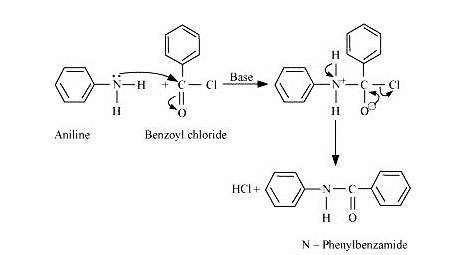
When aniline is treated with benzoyl chloride in the presence of base it gets converted into N-Phenylbenzamide.
New answer posted
6 months agoContributor-Level 10
5.32
Uses of Emulsions-
It is used in making of medicines,
Cleansing action of soaps is based on this emulsion
Digestion of fats in intestine takes place by the process of
Antiseptics and disinfectant added to water form emulsion for
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
