Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
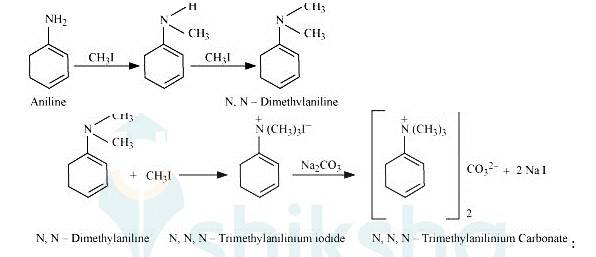
On excessive alkylation with methyl iodide aniline gets converted into N, N-Trimethylanilinium iodide. After reacting it with sodium carbonate it get converted into N, N-Trimethylanilinium carbonate.
New answer posted
6 months agoContributor-Level 10
8.11 (i) The atomic number of Cr is 24 and the electronic configuration is [Ar] 3d54s1. When 3 electrons are removed, it becomes Cr3+. The electronic configuration of Cr3+: 1s2 2s2 2p6 3s2 3p6 3d3 Or [Ar]3d3
(ii) The atomic number of Pm is 61 and the electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f5 5s2 5p6 6s2 or [Xe] 4f56s2. When 3 electrons are removed, it becomes Pm+3, having the electronic configuration,
Pm3+: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f4
Or, [Xe]544f4
(iii) The atomic number of Cu is 29 and has the electronic configuration of [Ar] 3d104s1. On removing one electron, the Cu+
New answer posted
6 months agoContributor-Level 10
5.31
Electrophoresis: The movement of colloidal particles under an applied electric potential is called When an electric potential is applied to two platinum electrodes dipping in colloidal solutions the colloidal particle move towards the Oppositely charged electrodes.
Coagulation: The process of settling of colloidal particles is called coagulation. When the charge is removed from colloidal solution somehow, then particle start coagulation and settling due to the force of
Dialysis: It is a process of removing a dissolved substance from AC colloidal solution by means of diffusion through a suitable the animal membrane or parchment paper
New answer posted
6 months agoContributor-Level 10
5.30
The catalytic reaction that depends upon the pore structures of the catalysts and the size of reactant and product molecules is called shape selective catalysis. Zeolites are good shape- selective catalysts.
New answer posted
6 months agoContributor-Level 10
8.10 The 5f orbitals (in actinoids) have a poorer shielding effect than 4f orbitals (in lanthanoids). Thus, the effective nuclear charge experienced by electrons in outer shells in case of actinoids is much more that experienced by lanthanoids.
As the effective nuclear charge experienced is high, the electrons are attracted with much force, hence the size of the atom decreases. Hence, actinoid contraction is greater from element to element than lanthanoid contraction.
New answer posted
6 months agoContributor-Level 10
5.29
Catalysis by zeolites is dependent on shape. Because zeolites are shape-selective catalysts. They are alumino silicates which are microporous in nature. It has Honeycomb structure. That makes them shape selective. In zeolites, some si atoms are replaced by Al to form Al-O-Si network.
Reactants are very sensitive to the pore size of zeolites. Zeolites are used in petrochemical industry. Ex- ZSM-5 used to convert alcohol into gasoline.
New answer posted
6 months agoContributor-Level 10
(i) CH3CH2CH2NH2 + HCl → CH3CH2CH2N+H3Cl-
The final product is (N-propyl ammonium chloride.)
(ii) (C2H5)3N + HCl → (C2H5)3N+HCl-
The final product is (Tri ethyl ammonium chloride)
New answer posted
6 months agoContributor-Level 10
5.28
Activity - It is the ability of the catalyst to accelerate the Reaction. It mostly depends upon the Chemisorption strength.
Selectivity - It is an ability to direct reaction to yield of a particular product i.e., One catalyst cannot be a catalyst for other reactions.
New answer posted
6 months agoContributor-Level 10
8.9 The stability in aqueous condition depends on the hydration energy of the ions when they bond to the water molecules. And, the hydration energy is the amount of heat released as an ionic substance is dissolved and its constituent ions are hydrated or surrounded by water molecules.
Now, in Cu2+ and Cu+ ion, Cu2+ has a greater charge density than the Cu+ ion and so forms much stronger bonds releasing more energy. Therefore, in an aqueous medium, Cu2+ ion is more stable than Cu+ ion. This is because the energy required to remove one electron from Cu+ to Cu2+, is compensated by the high hydration energy of Cu2+.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
