Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
(i) Carbylamine reaction
Carbylamine reaction is used as a test for the identification of primary amines. When aliphatic and aromatic primary amines are heated with chloroform and ethanolic potassium hydroxide, carbylamines (or isocyanides) are formed. These carbylamines have very unpleasant odours. Secondary and tertiary amines do not respond to this test.
For example,
(ii) Diazotisation
Aromatic primary amines react with nitrous acid (prepared in situ from NaNO2 and a mineral acid such as HCl) at low temperatures (273-278 K) to form diazonium salts. This conversion of aromatic primary amines into diazonium salts is known as diaz
New answer posted
6 months agoContributor-Level 10
Primary amine: A primary (1°) amine is an amine that has the following general structural formula. R= alkyl or aryl group Secondary amine: A secondary (2°) amine is an amine that has the following general structural formula. R1 and R2= alkyl or aryl group Tertiary amine: A tertiary amine is an amine that has the following structure R1, R2 and R3 are alkyl or aryl groups Identification of Primary, Secondary and Tertiary amines Primary, secondary and tertiary amines can be identified by the following test: Hinsberg's test: This is an excellent test for the identification of primary, secondary and tertiary amines. In this test, the amine is shaken with benzenesulphonyl chloride ( Hinsberg's reagent) in the presence of an excess of aqueous KOH solution when (i) A primary amine gives a clear solution which on acidification gives an N-alkylbenzene sulphonamide which is soluble in Due to the presence of strong electron withdrawing sulphonyl group in the sulphonamide, the H-atom attached to nitrogen can be easily released as a proton. So it is acidic and dissolves in alkali. (ii) A secondary amine reacts with Hinsberg's reagent to give a sulphonamide which is soluble in There is no H-atom attached to the N-atom in the sulphonamide Therefore it is not acidic and soluble in alkali. (iii) A Tertiary amine does not react with Hinsberg's reagent at all |
New answer posted
6 months agoContributor-Level 10
7.17
(i) C2H4 + O2→ 2CO2 + 2H2O
Ethane in reaction with oxygen gives carbon dioxide and water.
(ii) 4Al + 3 O2→ 2Al2O3
Aluminium on reaction with oxygen gives alumina.
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
8.16 (i) Vanadate (VO3 -)-Oxidation state of V is +
(ii) Chromate (CrO4 2-)-Oxidation state of Cr is +
(iii) Permanganate (MnO4 -) Oxidation state of Mn is + 7
New answer posted
6 months agoContributor-Level 10
Pkb value is the negative logarithm of the basicity constant (Kb) .i.e., pKb = -logKb
Evidently, smaller the value of pKb , stronger is the base (strong tendency to donate electrons). Aliphatic amines(R-NH2) are more basic(tendency to donate electrons) than aromatic amines(C6H5NH2) because of the following reasons:
=>In aliphatic amines, alkyl groups are present. Alkyl groups are electron releasing groups, hence they increase the elecron density of N-atom and thus is easily available to donate electrons. This poperty makes aliphatic amines more basic.
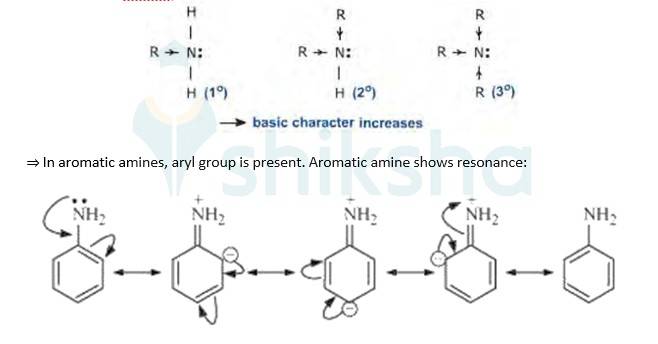
As a result of resonance, the lone pair of electrons on the nitrogen atom gets delocaliz
New answer posted
6 months agoContributor-Level 10
7.16
Pt is a noble metal (inert) and does not react with oxygen, whereas Zn, Ti, and Fe react with quickly with oxygen.
New answer posted
6 months agoContributor-Level 10
7.15
The oxygen atom has a smaller size and greater electronegativity than sulphur atom, hence intermolecular hydrogen bonding is possible in water (H2O). But, only weak Van there Waal's force holds H2S molecules, hence water is liquid and H2S is a gas.
New question posted
6 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers