Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
5.9
Adsorption- The accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed as adsorption. In this substance accumulate at surface only and does not penetrate into the adsorbent (material on the surface of which adsorption takes place).
Ex- If gases like O2, H2, NH3 taken in a closed vessel containing charcoal then gases get adsorbed on the surface of charcoal and reduce the pressure.
Absorption- In this molecules get distributed uniformly throughout the material Instead of accumulating at the surface.
Ex- When gas like O2 is mixed with water then O2 get distributed throughout the solve
New answer posted
6 months agoContributor-Level 10
5.8
The precipitate which is obtained from a chemical reaction always contain some unwanted substances (eg ions, impurities) which get adsorbed onto the surface of the precipitate.
Therefore, it becomes important to wash the precipitate before estimating it quantitatively so as to remove these unwanted adsorbed substances and obtain accurate results.
New answer posted
6 months agoContributor-Level 10
4.28 Given:
Time t = 40 min
When 30% decomposition is undergone, 70% is the concentration.
We know, time taken
t= 2.303/K log R0 / R
Where, k- rate constant
[R]0 - initial concentration
[R] - concentration at time 't'
40 = 2.303/K log R0 / 0.7 R0
40 = 2.303/K log 1 / 0.7
40 / 0.1549 = 2.303 / k
?258.23 = (2.303/k)
We know, Half-life t1/2 = 0.693/k
Which can be written as, t1/2 = 0.3010 * (2.303/k)
? t1/2 = 0.3010 * 258.23
? t1/2 = 77.72 min
New answer posted
6 months agoContributor-Level 10
4.27 Let, initial concentration be [R]°
Concentration at 90% completion be (100-90)/100)* [R]°
? Concentration at 90% be 0.1 [R]°
Concentration at 99% completion be (100-99)/100)* [R]°? Concentration at 99% be 0.01 [R]°
we know time, t= 2.303/K log R0 / R
Time taken for 90% completion is
T90 = 2.303 / K log R0 / 0.1 R0
T90 = 2.303 / K log 1 / 0.1
T90 = 2.303 / K log 10 / 1
T90 = 2.303 / K
Time taken for 99% completion is
T99 = 2.303 / K log R0 / 0.01 R0
T99 = 2.303 / K log 1 / 0.01
T99 = 2.303 / K log 100 / 1
T99 = 2 X 2.303 / K
T99 = 2 T90
Hence, the time taken to complete 9% of the
New answer posted
6 months agoContributor-Level 10
4.26 Initial concentration, [R]° = 1? g
Final concentration, [R] Half-life t1/2 = 28.1 years Solution:
We know, t1/2 = 0.693/k Where, k – rate constant
? k = 0.693/ t1/2
? k = 0.693/ (28.1 yrs)
? k = 0.0246 yrs-1
Also, t = 2.303 / k log R0 / R
If t = 10yrs, then, using the formula, we get,
t = 2.303 / k log R0 / R
10 = 2.303 / 0.0246 log 1 / R10
log 1 / R10 = 0.0246 X 10 / 2.303
log 1 / R10 = 0.246 / 2.303
1 / R10 = antilog (0.246 / 2.303)
1 / R10 = 1.278
? R10 = 0.7824? g
If t = 60yrs, then again, we get,
60 = 2.303 / 0.0246 log 1 / R60
log 1 / R60 = 0.0246 X 60 / 2.303
log 1 / R60 =
New answer posted
6 months agoContributor-Level 10
4.25 Given:
Order of the reaction = 1
Let, Initial concentration [R]°= x
Final concentration [R] = x/16
Rate constant k = 60 s-1
We know, time
t= 2.303 / k log R0 / R
t = 2.303 / 60 log (x/x/16)
t = 2.303 / 60s-1 log (1/1/16)
t = 2.303 X log 16 / 60s-1
Solving, we get t = 4.6 * 10-2s
New answer posted
6 months agoContributor-Level 10
4.24
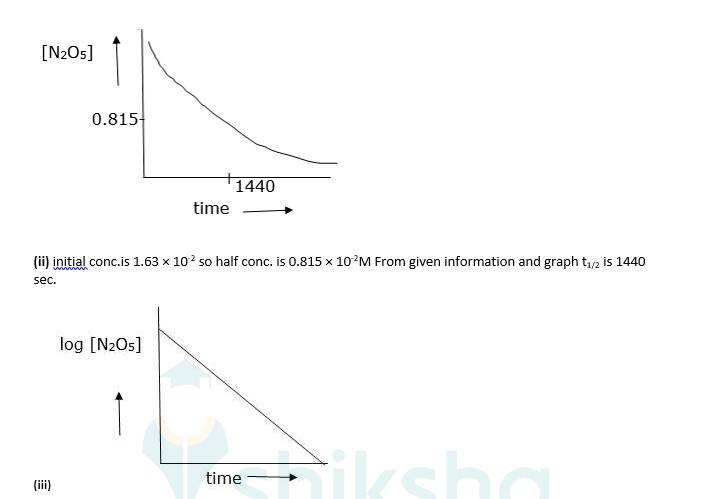
(iv) As log [N2O5] vs time is a straight line given reaction is first Hence its rate law will be, Rate = k [N2O5]
(v) The slope of above graph is slope = 0.000209 K = 303 * slope
⇒4.82 * 10-4sec-1
Now, t1/2 = 0.693/K.
⇒0.693/4.82 * 10-4
⇒t1/2 = 1438 sec. which is almost equal to (ii)
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
4.23 Radio active decay occurs via first order rate law,
t1/2 = 5730 years. rate constant (k) of given decay is 0.693/t1/2
⇒0.693/5730 = 1.2 * 10-4 year-1
By first order integrated rate law age of the sample will be,
T = ( 2.303 / 1.2 X 10-4 ) log (A0/At)
where T is the age of the sample, A0 is the initial activity of the sample. and At is the activity of the sample at any time t
T = ( 2.303 / 1.2 X 10-4 ) log (A0/0.8 A0)
T = 0.18 * 104 years.
Age of given sample is 0.18 * 104 years.
New answer posted
6 months agoContributor-Level 10
4.22 Half life of first order reaction is, t1/2 = ln2/K where t1/2 is half life of first order reaction, K is rate constant of First order reaction.
(i) t1/2 = ln2/200 s-1
⇒t1/2 = 0.693/200 s-1 (? ln2 = 0.693)
⇒t1/2 = 0.003465 sec.
(ii) t1/2 = ln2/2 min-1
⇒t1/2 = 0.693/2 min-1 (? ln2 = 0.693)
⇒t1/2 = 0.3465 min
(iii) t1/2 = ln2/4 year-1
⇒t1/2 = 0.693/4 years -1 (? ln2 = 0.693)
⇒t1/2 = 0.17325 year.
Half life of 3 reactions are 0.003465 sec, 0.3465 min, 0.17325 year, respectively.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers
