Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
4.21 As reaction is first order with respect to A and zero Order with respect to B. Then changing the concentration of B won't affect the rate of reaction and increasing concentration of A 'n' times will increase the rate by 'n' times. By this logic lets fill the table- In first blank space concentration of A will be 0.2 mol L-1 because the rate is doubled. In second blank space, Rate will be 8 * 10-2mol L-1min-1 because the concentration of A is increased 4 Times. In third blank space concentration of A will be 0.1 mol L-1 because the rate is same as in experiment I.
Experiment | [A]/mol L-1 | [B]/mol L-1 | Initial rate/mol L-1 min-1 |
I | 0.1 | 0.1 | 2.0 * 10-2 |
II | 0.2 | 0.2 | 4.0 * 10-2 |
III | 0.4 | 0.4 | 8.0 * 10-2 |
IV | 0.1 | 0.2 | 2.0 * 10-2 |
New answer posted
6 months agoContributor-Level 10
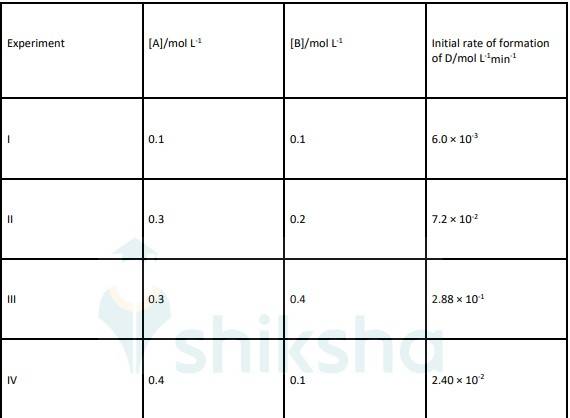
4.20 By comparing Experiment I and IV if we increase the concentration of A by 4 times then Rate also increased by 4 times. That means order with respect to A is 1.
By comparing Experiment II and III if we double the concentration of B Rate increases by 4 times that means order with respect to B is 2.
Rate law of reaction will be, Rate = k [A] [B]2
To find K, K = rate/ [A] [B]2 i.e. K = 6.0 * 10-3/ [0.1] [0.1]2
K = 6 mol-2L2sec-1
Order with respect to A and B is 1 and 2 respectively. And value of K (rate constant) is = 6 mol-2L2sec-1
New answer posted
6 months agoContributor-Level 10
4.19 When concentration of B is changed then rate of reaction doesn't change that means order with respect to B is 0. But when the concentration of A is doubled rate increased by 2.82 times i.e.21.5 = 2.82.Hence order with respect to A is 1.5.
Order with respect to A and B is 1.5 and 0, respectively.
New answer posted
6 months agoContributor-Level 10
4.18 (i) Order is power raised to reactant in rate law, hence,
Rate = k [A] [B]2
(ii) When the concentration of B is increased three times then the rate is affected by the square of The rate is increased by 9 Times.
(iii) When the concentration of reactant both A and B is doubled then the rate will have affected as square of reactant B and Two times of Reactant Overall increase in rate is 8 times
(a) When the concentration of B is increased by three times, the rate is increased by nine times
(b) When of both reactants is doubled, the Rate increases 8 times.
New answer posted
6 months agoContributor-Level 10
4.17 (i) Average rate of reaction over interval is [change in concentration]/ [time taken] e.
[0.31 - 0.17] / [60-30] = 0.00467 mol L-1 sec-1
(ii) the pseudo first-order rate constant can be calculated by K = (2.303/t) log (Ci/Ct)
where K is Rate constant,
t is time taken,
Ci is initial concentration
Ct is Concentration at time t.
K = (2.303/30) log (0.55/0.31)
? K = 1.9 * 10-2 sec-1
(i) Average rate between 30 to 60 sec is 0.00467 mol L-1sec-1
(ii) Pseudo first order rate constant is 1.* 10-2sec-1
New answer posted
6 months agoContributor-Level 10
5.7
According to Hardy-Schulze law
'The greater the valence of the flocculating ion added, the greater is its power to cause precipitation.
As this law takes into consideration of only the charge present on the ion and not the size of the ion. So when the size of the atom is considered, smaller the size of an atom more will be its polarising power.
So Hardy-Schulze can be modified in terms of the polarising power of the flocculating ion as 'The greater the polarising power of the flocculating ion added, the greater is its power to cause precipitation.
New answer posted
6 months agoContributor-Level 10
4.16 Increase in temperature increases the rate constant of a reaction. as we know increase in temperature increases the rate of reaction to satisfy the equation Rate = k [concentration]n where n can be any real number. k have to increase as concentration is almost not changing over small temperature change.
Increasing the temperature by 10°c almost doubles the rate constant. This can be represented quantitatively by the help of the Arrhenius equation-
K = Ae-Ea/RT, where k is rate constant, Ea is the activation energy, R is the universal gas constant, T is the absolute temperature.
New answer posted
6 months agoContributor-Level 10
5.6
Desorption is a process in which substance (reactant + product) is released from the surface which is the opposite process of sorption.
The role of desorption in the process of catalysis is to make the surface of the solid catalyst-free for fresh adsorption of reactants on the solid surface for further reactions to take place.
New answer posted
6 months agoContributor-Level 10
The complex is an anion with chromium as central atom, 2 water molecules and 2 oxolate ions with -2 negative charge Balance overall charge as 0, we get oxidation state of Cr as:
X + 2 (0) + 2 (-2) = -1 X = + 3.
Name of compound: potassium diaquadioxolatochromate (III) trihydrate. Electronic configuration of Cr: 3d3, t2g3
New answer posted
6 months agoContributor-Level 10
4.15 Let suppose reaction
A? B; having rate law, Rate = K [A]2
(i) If the concentration of A is doubled then the rate will affect by the square of concentration i.e. rate will become 22 = 4 times.
(ii) If the concentration of A is halved, then the rate will affect the square of the concentration i.e (1/2)2 = 1/4
(i) Rate becomes 4 times of initial Rate (ii) Rate becomes 1/4 times of initial Rate
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers