Polymers
Get insights from 155 questions on Polymers, answered by students, alumni, and experts. You may also ask and answer any question you like about Polymers
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
15.2
Polymers are classified based on structure, into 3 types:
Linear Polymer: They have a long and straight chain of Ex: high-density Polythene, Polyvinyl chloride

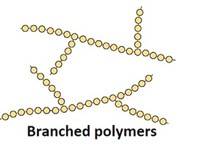
Branched-chain Polymers: They have linear molecular chains along with some Ex: less density polythene.
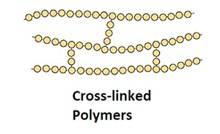
Cross-linked or network Polymers: In these polymers, strong covalent bonds are between the linear chains. Generally, they contain 2 or 3 types of functional groups. Ex: Bakelite, melamine.
New answer posted
8 months agoContributor-Level 10
15.1
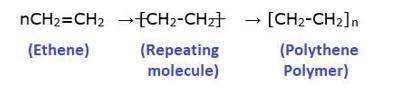
Polymer=Poly + Mer
Poly means “many” and “Mer” means unit or part. A polymer is a large molecule which is formed by linking repeating structural units. The structural units are generally simple molecules and they are linked by a covalent bond to form a polymer.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
