Solutions
Get insights from 202 questions on Solutions, answered by students, alumni, and experts. You may also ask and answer any question you like about Solutions
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iii) Ar< CO2< CH4< HCHO
Explanation: The higher the KH value, the better. The solubility of a gas at a given pressure will be lower, hence the solubility of given gases will increase as KH values rise.
New question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) At specific composition methanol-acetone mixture will form minimum boiling azeotrope and will show positive deviation from Raoult's law.
Explanation: (i) (A-A) or (B-B) interactions are more powerful than (A-B) interactions, where A represents a methanol molecule and B represents an acetone molecule. It means that molecules of A (or B) will have an easier time escaping from this solution. This will result in a positive departure from Rault's law when the vapour pressure rises.
(ii) The methanol-acetone mixture forms the smallest boiling azeotrope due to this
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iv) 0.016
Explanation: Apply the relation : M1V1= M2V2
Given: M1=0.02M, V1=4L, M2=? V2=5L
Therefore, 0.02*4L=M2*5L
M2=0.08/5
=0.016 M
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iv) A–B interactions are weaker than those between A–A or B–B.
Explanation: (i) At a given composition, the solutions that demonstrate a big positive divergence from Rault's law form a minimum boiling azeotrope.
(ii) When Rault's law is deviated positively, A-B interactions are weaker than A-A or B-B interactions.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
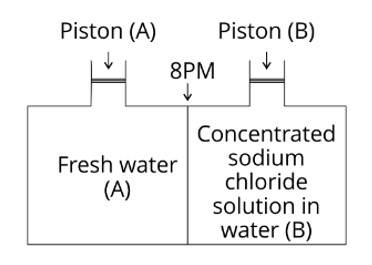
(i) Vapour pressure in container (A) is more than that in container (B).
Explanation: Due to the fleeing inclinations of water molecules from the liquid's surface, the vapour pressure rises. The vapour pressure increases as the number of molecules on the liquid's surface increases. Because only water molecules are present at the surface of beaker A, it has a higher vapour pressure. However, a fraction of the surface area of the solution in beaker B containing NaCl solution is occupied by NaCl molecules, which are non-volatile and have no tendency to escape. As a resul
New answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) Solution (A) will follow Raoult's law.
Explanation: A-A and B-B intermolecular interactions should be almost identical to A-B type interactions in an ideal solution.
New question posted
8 months agoNew question posted
8 months agoNew answer posted
8 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) Water will move from side (B) to side (A) if a pressure greater than osmotic pressure is applied on piston (B).
Explanation: Due to reverse osmosis, water will travel from side (B) to side (A) if a pressure greater than osmotic pressure is applied to the piston (B).
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers