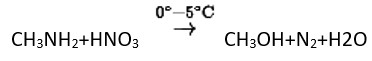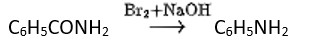Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Get insights from 127 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Thirteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (C)
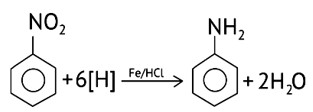
Using active metals like iron in acidic conditions can be efficiently used for the reduction of nitro compounds.
Under the acidic conditions, the intermediate compounds that may form are readily reduced to primary amine and pure product of primary amine is obtained.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (C)
In the process of nitration of benzene, firstly H2SO4 dissociates into H+ and HSO4−. The proton produced further reacts with HNO3 to give a positively charged complex that is unstable and dissociates into nitronium ions and water as products. The nitronium ion is an electron-deficient species i.e. electrophile that attacks the electron cloud of the benzene ring.
H2SO4 (conc.) ? H++HSO4−
H++HNO3? H2NO3+
H2NO3+? NO2+ + H2O
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
Primary aliphatic amines react with nitrous acid to form aliphatic diazonium salts, which are unstable and release nitrogen gas and alcohol quantitatively.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (C)
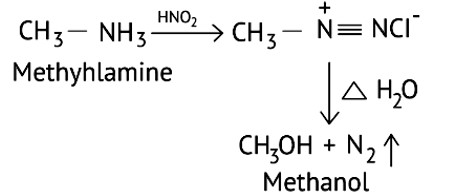
Methyl amine reacts with HNO3 form methanol with release of nitrogen gas and water as side products.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
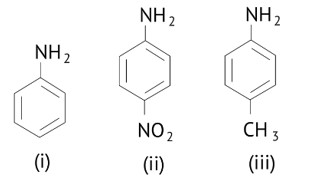
The greater the electron density towards the ring, the greater its basic strength.
The electron withdrawing group reduces basic strength, whereas the electron donating group increases basic strength.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
Hoffmann invented a method for producing primary amines by treating an amide with bromine in an aqueous or ethanolic sodium hydroxide solution. An alkyl or aryl group migrates from the amide's carbonyl carbon to the nitrogen atom during this degradation reaction.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (B)
Hoffmann bromamide reaction- it is also called degradation reaction as in this reaction primary amide group is treated with halogen first (Br) then the halogen-substituted amide product is converted to a primary amine with the release of carbon dioxide gas.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
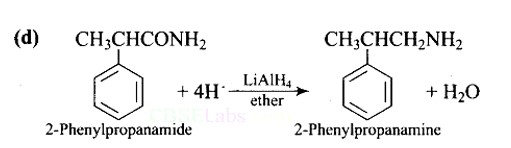
Lithium aluminium hydride in ether is a strong reducing agent that donates its hydride ion to any C=O containing functional groups into corresponding reduced compounds. Here, the amide group is converted to an amine functional group.
LiAlH4 in ether is the best reagent for converting 2-phenylpropanamide to 2-phenylpropanamine.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: ( C)
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Ans: (D)
Gabriel synthesis reaction:
Gabriel's synthesis is a method for producing primary amines. When phthalimide is treated with ethanolic potassium hydroxide, it forms a potassium salt of phthalimide, which when heated with an alkyl halide and then alkaline hydrolyzed yields the corresponding primary amine. This method cannot produce aromatic primary amines because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers