Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
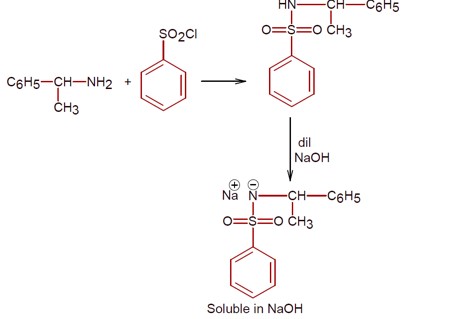
Only 1° amine react with benzene sulphonyl chloride to give a compound which is soluble in alkali
New answer posted
3 months agoContributor-Level 10
Iron (III) iodide (FeI? ) does not exist because it is unstable. The Fe³? ion is a strong enough oxidizing agent to be easily reduced to Fe²? by the I? ion, which in turn is oxidized.
New answer posted
3 months agoContributor-Level 9
Metal sulphide sols are negatively charged while metal oxide sols are positively charged sols. So, CdS is negative while TiO? is positively charged sol.
New answer posted
3 months agoContributor-Level 10
In sodium hydride (NaH), hydrogen has an oxidation state of -1. In this state, it can only act as a reducing agent.
New answer posted
3 months agoContributor-Level 10
Colloidal particles are small enough to pass through an ordinary filter but can be stopped by an ultrafilter paper due to their specific particle size range.
New answer posted
3 months agoContributor-Level 10
The atomic numbers and classifications for the given elements are: As (Arsenic, atomic no. 33) is a metalloid, I (Iodine, atomic no. 53) is a non-metal, and Bi (Bismuth, atomic no. 83) is a metal.
New answer posted
3 months agoContributor-Level 10
In the ammonolysis reaction, HCl is produced as a byproduct. To neutralize this acidic impurity, the mixture is treated with NaOH.
New answer posted
3 months agoContributor-Level 9
First ionization enthaly of Mg is smaller than Ar and Cl but higher than Na.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers