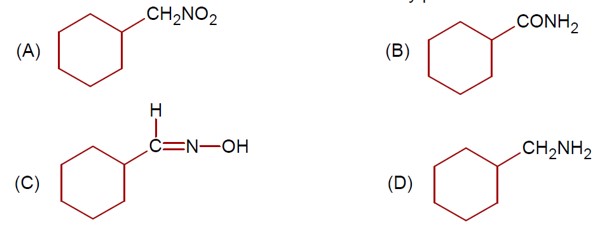
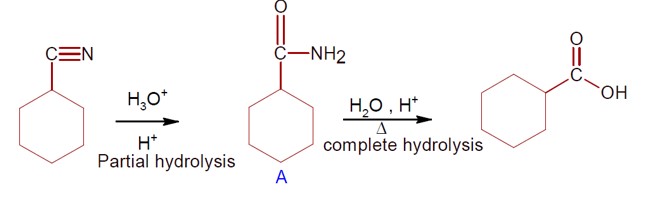
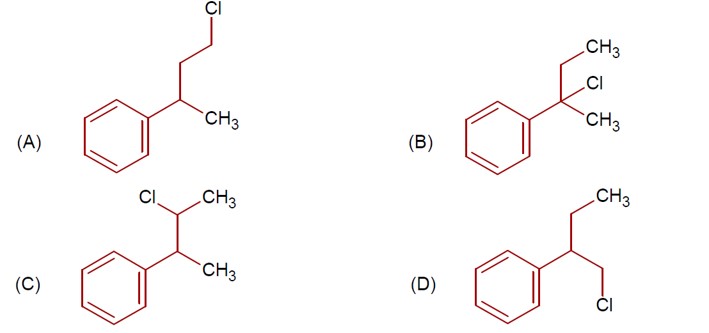
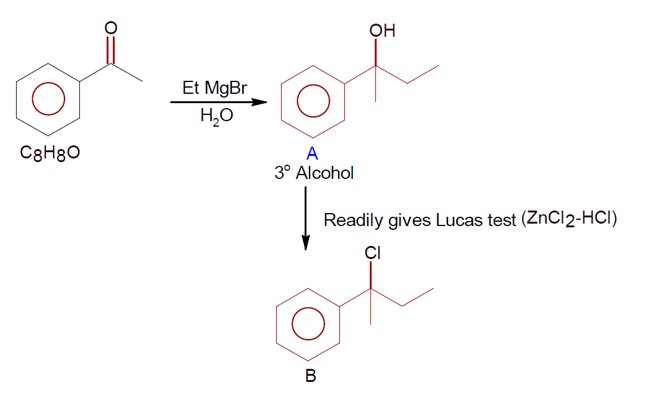
Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
3-Hydroxy propanal
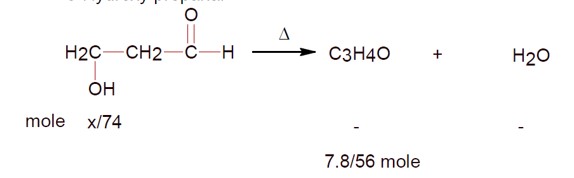
If 7.8g of C? H? O (molar mass 56 g/mol ) is formed, calculate the initial weight of 3-hydroxy propanal (molar mass 74 g/mol ).
Weight = (7.8/56) * 74 * (100/64) [Assuming 64% yield, though the number seems out of place].
Ans ≈ 16 g.
New answer posted
3 months agoContributor-Level 9
AX is a diatomic molecule with a bond order of 2.5.
The compound is NO. The total number of electrons = 15 (7+8).
New answer posted
3 months agoContributor-Level 9
For an acidic buffer solution, pH = pKa + log ( [Base]/ [Acid]).
Given pH = 5.74 and pKa = 4.74.
5.74 = 4.74 + log ( [Base]/1).
1 = log ( [Base]).
[Base] = 10M.
New answer posted
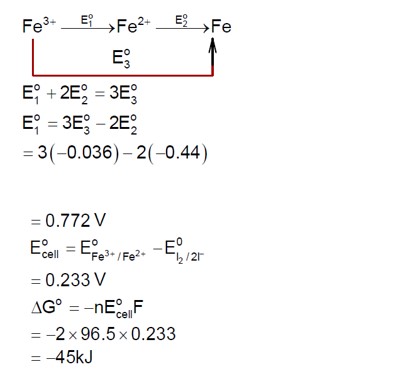
3 months agoContributor-Level 9
For the reaction C? H? → C? H? + H? , calculate the enthalpy change (ΔH).
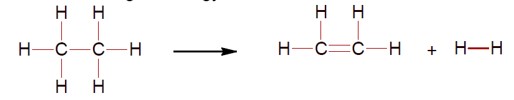
ΔH = [Bond energy (C-C) + 6 * Bond energy (C-H)] - [Bond energy (C=C) + 4 * Bond energy (C-H) + Bond energy (H-H)]
ΔH = 347 + 2 (414) - 611 - 436 = 128 kJ/mol.
New answer posted
3 months agoContributor-Level 9
CH? (methane) is produced / generated from paddy fields. And methane leads to both global warming and photochemical smog.
CO? is used in photosynthesis, acid rain etc. but methane is not consumed.
So methane is a stronger global warming gas than CO?
New answer posted
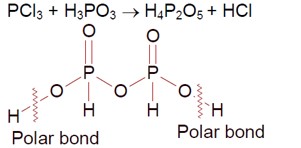
4 months agoContributor-Level 9
Phosphorous Trichloride + Phosphorous acid
O-H bond is polar, so the number of ionisable hydrogens are 2.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers