Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
For concentration cell E? cell = 0
Anode: Cu (s) → Cu²? (aq)?
Cathode: Cu²? (aq)? → Cu (s)
Overall: Cu²? (aq)? → Cu²? (aq)?
As ΔG = −nFEcell
If ΔG = -ve than Ecell is positive
Ecell = E? cell − (0.059/2) log (C? /C? )
Ecell = − (0.059/2) log (C? /C? )
Ecell > 0 ⇒ C? > C?
New answer posted
4 months agoContributor-Level 10
Relative lowering in vapour pressure depends on no. of mole of solute greater the no. of mole of solute greater in RLVP and smaller will be vapour pressure. So order of vapour pressure is B > C > A.
New answer posted
4 months agoContributor-Level 10
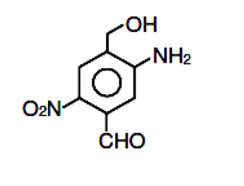
The chemical structure shown is 5-Amino-4- (hydroxymethyl)-2-nitrobenzene carbaldehyde.
New answer posted
4 months agoContributor-Level 10
(i) Ca (OH)? is used in white wash.
(ii) Plaster of paris is used in making of molds for plaster statues.
(iii) NaCl is used in preparation of washing soda.
(iv) A suspension of Mg (OH)? in water is used in medicine as an antacid under name of milk of magnesia.
New answer posted
4 months agoContributor-Level 10
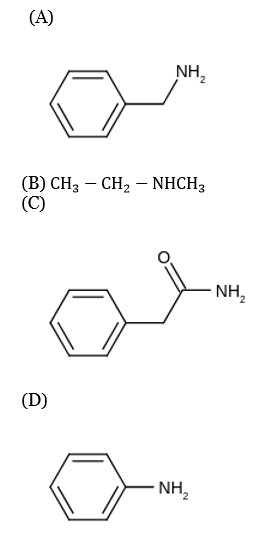
(I) Lucas reagent → Only ZnCl? /Conc. HCl
(II) Dumas method → CuO/Δ
(III) Kjeldahl's method → Conc. H? SO? /Δ
(IV) Heinsberg reagent → C? H? SO? Cl/ aq. NaOH
New answer posted
4 months agoContributor-Level 10
For d? configuration if Δ? < P the electronic configuration is t? g eg
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
In the ccp lattice of oxide ions effective number of O? ² ions = 8 *? + 6 * ½ = 4
In the ccp lattice,
No. of octahedral voids = 4
No. of tetrahedral voids = 8
Given M? atoms occupies 50% of octahedral voids and M? atoms occupies 12.5 of tetrahedral voids
No. of M? metal atoms = 4 *? /? = 2
No. of M? metal atoms = 8 * ¹²·? /? = 1
∴ Formula of the compound = (M? )? (M? )O?
∴ Oxidation states of metals M? & M? respectively are +2 and +4 .
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers