Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
For A
0.693/300 = (2.303/t) log (A? /A? )
For B
0.693/180 = (2.303/t) log (B? /B? )
Given A? = B? & A? = 4B?
Substituting & solving we get t = 900 s
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 10
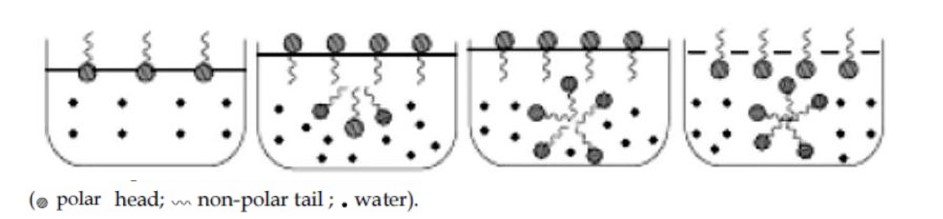
At CMC, the particles cluster together through lyophobic end to form associated colloid called micelle. Further all lyophilic ends (polar head) get projected towards water.
New answer posted
4 months agoContributor-Level 10
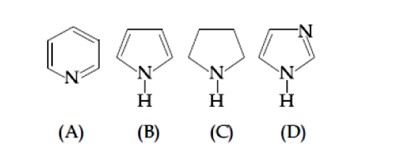
B is least basic as lone pair of electron is present in resonance so as to make the system aromatic in nature
D is most basic as it results in formation of equivalent resonating structures upon attack of H?
Among A and C, the former is less basic as sp² hybridistion of nitrogen decrease its basic strength.
Hence option 3 follows
New answer posted
4 months agoContributor-Level 10
According to Aufbau's principal, the increasing energy of atomic orbitals for sixth period element follows the order
6s < 4f < 5d < 6p
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 8
The major functions of hormones are:
- Regulating Metabolism
- Controlling Growth and Development
- Managing Stress and Energy
- Regulating Sleep and Mood
New answer posted
4 months agoContributor-Level 8
Hormones present in the human body are:
- Thyrotropin-releasing hormone (TRH)
Dopamine (prolactin-inhibiting hormones)
Gonadotropin-releasing hormone (GnRH)
Adrenal Glands
Androgens
Estrogen
Inhibin
Testosterone
Gastrin
Melatonin
New answer posted
4 months agoContributor-Level 8
Below is the list of hormones:
- Cortisol
- Estrogen & Progesterone
- Aldosterone
- Insulin
- Growth Hormone
- Glucagon
- Adrenaline (Epinephrine)
- Thyroxine (T4) and Triiodothyronine (T3)
- Melatonin
New answer posted
4 months agoContributor-Level 10
There are multiple factors that make the carbonyl group a strong ligand. Check the list below for the reasons.
- Unlike other alkyl ligands, it is an unsaturated compound.
- Due to its unsaturated nature, it has difficulty donating? electron density.
- It has a tendency to accept? (Pie) antibonding electrons.
- CO ligand acts as Lewis acid and donates a lone pair of electrons to form a metal-carbon bond.
- The? -acidic nature of CO gives a strong field and greater d-orbital splitting.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers