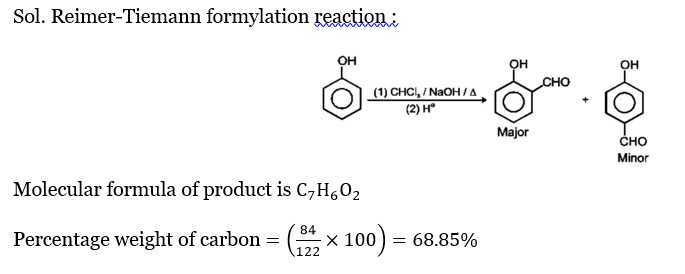
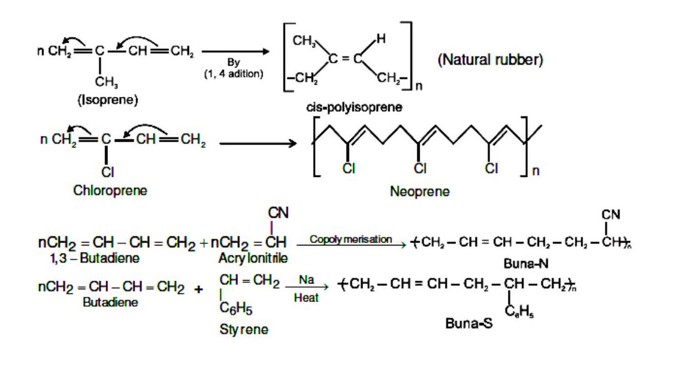
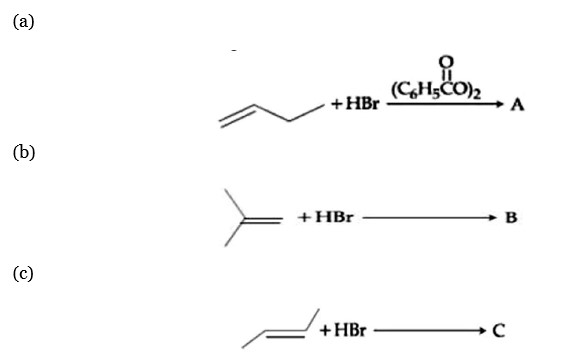
Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
To understand the phenomenon behind this, have a close look at the formula of the first order reaction.
t1/2 = 0.693/k
Here, we can see that the half life is only dependent on k (rate constant) since there is no [A]' in the formula. Hence, we can easily conclude that the half life of a first order reaction is independent of the initial concentration [A]'.
New answer posted
4 months agoContributor-Level 10
log (k? /k? ) = (Ea / 2.303R) [1/T? - 1/T? ]
log (3.555) = (Ea / (2.303R) [1/303 - 1/313]
1.268 * 8.314 * 303 * 313 = 10Ea
So, Ea = 100 kJ
New answer posted
4 months agoContributor-Level 10
According to IUPAC convention for naming of elements with atomic number more than 100, different digits are written in order and at the end ium is added. For digits following naming is used.
0 -nil
1-un
2-bi
3-tri
and so on.
New answer posted
4 months agoContributor-Level 10
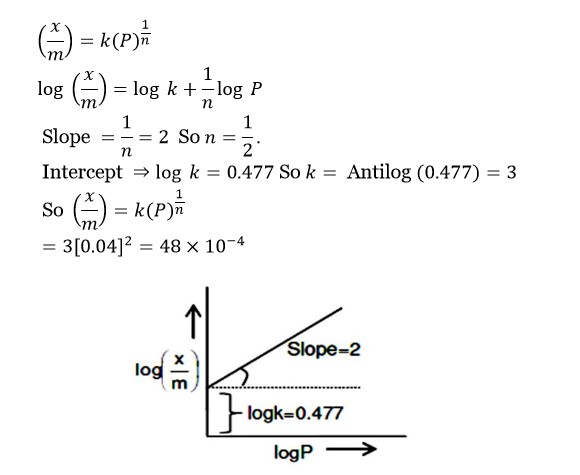
(x/m) = k (P)¹/?
log (x/m) = log k + (1/n) log P
Slope = 1/n = 2 So n = ½
Intercept ⇒ log k = 0.477 So k = Antilog (0.477) = 3
So (x/m) = k (P)¹/? = 3 [0.04]² = 48 * 10?
New answer posted
4 months agoContributor-Level 10
Misch metal consists of Lanthanide metal (? 95%) and iron (? 5%) and traces of S, C, Ca and Al.
New answer posted
4 months agoContributor-Level 10
Na? SO? + H? SO? → SO? + Na? SO? + H? O
SO? + 2NaOH → Na? SO? + H? O
Na? SO? + SO? + H? O → 2NaHSO?
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers