Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Excess of nitrogen and phosphorus is primarily responsible for eutrophication and hence an indicator of polluted environment.
New question posted
4 months agoNew answer posted
4 months agoContributor-Level 10
In endothermic reaction formation of reactants is favoured upon decrease in temperature. Addition of inert gas at constant volume and temperature has no effect on equilibrium.
New answer posted
4 months agoContributor-Level 10
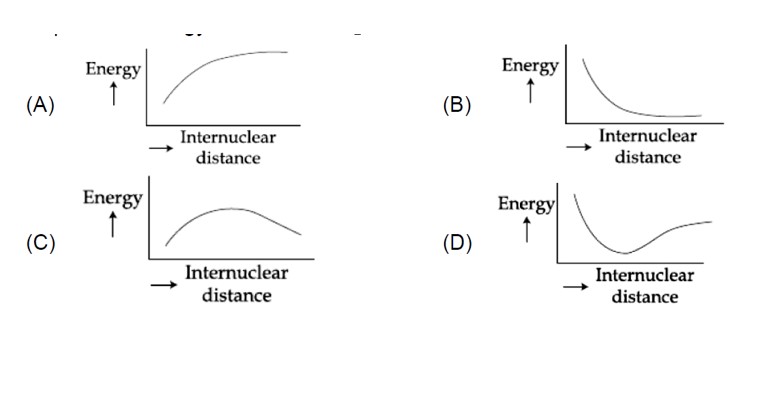
With decrease in inter-nuclear distance, the potential energy of the system decreases, reaches a minimum value and then sharply increases due to rise in inter-electronic as well as inter-nuclear repulsions
New answer posted
4 months agoContributor-Level 10
Among its different uses noradrenaline is used as an anti-depressant.
New answer posted
4 months agoContributor-Level 10
ρ = M/V
∴ 6.17 = (2 x MX? ) / (N? x (300 x 10? ¹? )³)
Solving MX? = 50 g
∴ No. of molecules in 200 g
= (200/50) x N? = 4 N?
New answer posted
4 months agoContributor-Level 10
In water gas shift reaction carbon monoxide is oxidized into carbon dioxide by treating it with steam in presence of catalyst.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers