Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
C? H? + 5O? → 3CO? + 4H? O
C? H? + (13/2)O? → 4CO? + 5H? O
∴ 2 mol C? H? is given
∴ 13 mol O? is required for combustion of Butane
∴ Total mol of O? = 5 + 13 = 18
New answer posted
4 months agoContributor-Level 10
Ellingham diagram provides information on Gibb's free energy for formation of oxides as a function of temperature.
New answer posted
4 months agoContributor-Level 10
Industries manily select an optimal temperature which is high enough to increase the reaction rate (molecules colliding with each other at higher fequencies) and at the same time is balanced to avoid any side effect. This effectively yields high quality production without any wastage or leading to harmful results while ensuring safety of the consumers too.
New answer posted
4 months agoContributor-Level 10
Gd: [Xe]4f?5d¹6s²
∴ Gd³? is [Xe]4f?
Also μ = √n (n + 2) B.M
= √7x9 B.M = 7.9 B.M
New answer posted
4 months agoContributor-Level 10
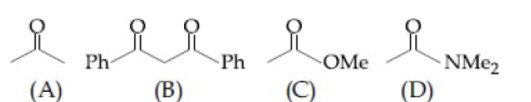
B is the most acidic as it is active methylene group. D is least acidic due to crossconjugation in conjugate base.
∴ Option 3 follows
New answer posted
4 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers