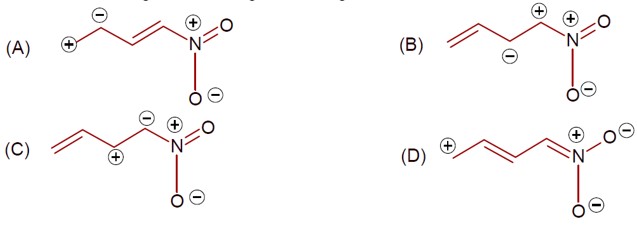Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
(x/m) = k (p)¹/? (1)
64 (x/m) = k (2p)¹/? (2)
From equation (1) and (2)
64 = 2¹/?
2? = 2¹/?
1/n = 6
n = 1/6
⇒ n = 0.167
= 16.7 * 10? ²
New answer posted
4 months agoContributor-Level 9
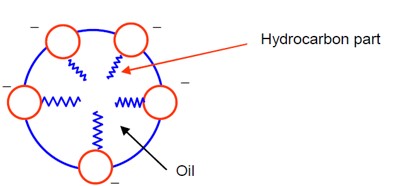
[Diagram of a micelle formed by sodium stearate in oil]
Spherical micelles forms and hydrocarbon part are water repelling and attracted by oily part.
New answer posted
4 months agoContributor-Level 10
? = CRT
2.42 * 10? ³ = ( (1.46/M_polymer) / 0.1 ) * 0.083 * 300
M_polymer = (1.46 * 0.083 * 300) / (2.42 * 10? ³ * 0.1)
= 14.96 * 10? gm = 15 * 10? gm
New answer posted
4 months agoContributor-Level 9
Oxidation states:
CrO? (+6), Fe?O? (+3), MnO? (+4), V?O? (+5), Cu?O (+1)
(a), (b), (c), (d), (e)
Order of oxidation numbers:
Cu?O < Fe?O? < MnO? < V?O? < CrO?. Hence correct order is; e < b < c < d < a
New answer posted
4 months agoContributor-Level 10
Organic compound + Cl? → A (Containing Cl)
5gm
A + AgNO? → AgCl
0.5 gm → 0.3849gm
Mole of AgCl = 0.3849 / 143.32
Mass of Cl = (0.3849/143.32) * 35.5 ≈ 0.095
% of Cl in A = (0.095/0.5) * 100 = 19%
New answer posted
4 months agoContributor-Level 10
In triethyl amine, nitrogen is sp³ hybridized, hence bond angle is approximately 109°28' but since lone pair- bond pair repulsion is greater than bp-bp repulsion. Hence, the exact angle is found to be 108°.
New answer posted
4 months agoContributor-Level 10
Number of GI in [Co (NH? )? (NO? )? ] is 2 i.e, X
Number of GI in [Cr (C? O? )? ]³? is 0 i.e. Y
[X + Y] = 2
New answer posted
4 months agoContributor-Level 10
Λ? = K * 1000 / M = (2 * 10? * 10³) / 10? ³ = 20 Scm²mole? ¹
For weak acid (α) = Λ? / Λ? = 20 / 190 = 2/19
K? = Cα² / (1 - α) = 10? ³ * (2/19)² / (1 - 2/19)
= 10? ³ * (4/361) / (17/19) = 0.01238 * 10? ³
= 12.38 * 10?
So, ans is 12 (the nearest integer).
New answer posted
4 months agoContributor-Level 9
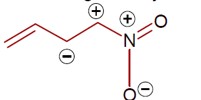
Same charge on adjacent atom is not stable. Hence the incorrect resonating structure is,
[Image of the incorrect resonance structure with adjacent positive charges]
New answer posted
4 months agoContributor-Level 10
In Aniline, lone pair is delocalized on less EN carbon atom while in acetamide it is delocalized on more EN oxygen atom. Hence aniline is more basic than acetamide.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers