Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
N > O > Be > B
Due to half filled configuration N has more I.E than oxygen and due to fully filled configuration Be has more I.E than B.
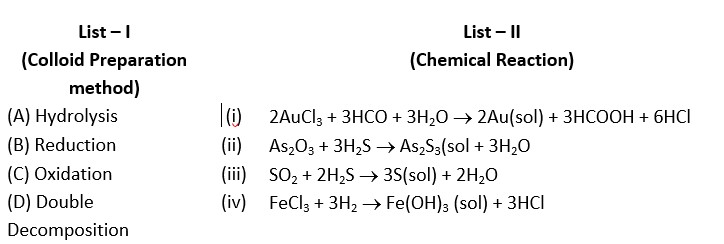
(a) → (ii) (b) → (iii) (c) → (iv) (d) → (i)
New answer posted
4 months agoContributor-Level 10
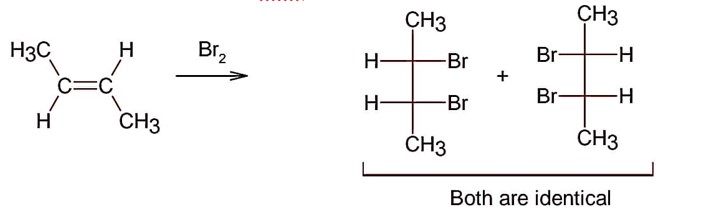
Electrophilic addition of bromine to an alkene is anti-addition, in which cis-alkene gives two enantiomers and trans – alkene gives meso form
Here; trans-but-2-ene will give meso products
New answer posted
4 months agoContributor-Level 10
Calamine is the ore of zinc i.e. ZnCO3.
Malachite is the ore of copper i.e. CuCO3.Cu (OH)2.
New answer posted
4 months agoContributor-Level 10
Electronic configuration of Fe is [Ar] 4s23d6 and in +3 oxidation state it has [Ar] 4s03d5 configuration.
New answer posted
4 months agoContributor-Level 10
K = 3.3 * 10-4 s-1
Time for 40% completion ; t
Using
3.3 * 10-4 =
so; the nearest integer is 26.
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
Mass of Na+ in 50 ml = 70 * 50 = 3500 mg
23000 mg of Na+ is present in 85000 mg of NaNO3 (1 mole NaNO3 contains 1 mole Na+)
mg Na+ will be present in
= 12934.78 mg
= 12.93478 gm
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers