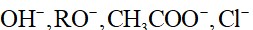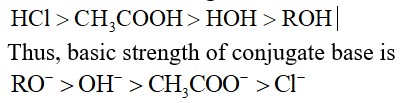Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Stronger the acid, weaker will be its Bronsted base.
Order of acidic strength:
New answer posted
3 months agoContributor-Level 10
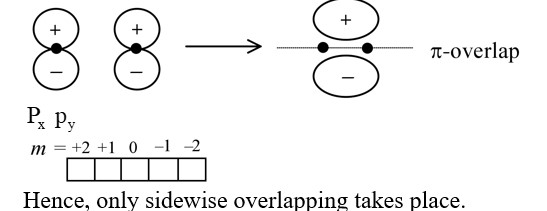
For overlap, the lobes of the atomic orbitals are perpendicular to the line joining the nuclei.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 8
Yes, NH? is a primary amine; it is also known as the amino group. Amines are organic compounds derived from ammonia in which one or more hydrogen atoms are replaced by alkyl or aryl groups.
New answer posted
3 months agoContributor-Level 8
Amines are bases because they have a lone pair of electrons on nitrogen and their nucleophilic nature (they are electron-rich, so they are attracted to electron-deficient species to donate electrons and accept protons.
New answer posted
3 months agoContributor-Level 8
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
New answer posted
3 months agoContributor-Level 10
Olefin is a general terms used for unsaturated hydrocarbons such as hydrocarbons which have more than one bond between their carbon atoms. It is derived from the latin word oleum (oil) and facere (make), since these products formed are usually oily in nature.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers