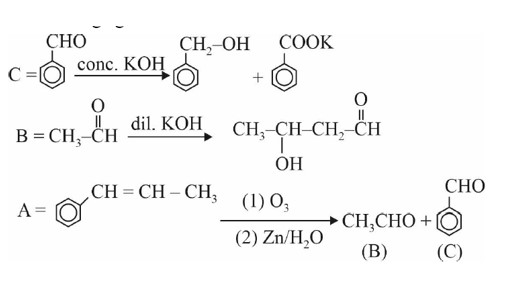
Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 9
Photochemical smog has high concentration of oxidising agent like and and is therefore called as oxidising agent.
New answer posted
3 months agoContributor-Level 9
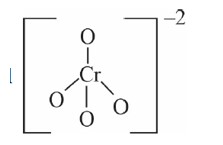
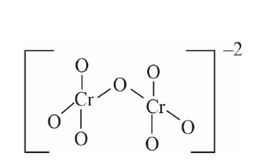
Chromate ion shape tetrahedral
But dichromate ion has only one common oxygen bond.
New answer posted
3 months agoContributor-Level 9
Above a particular concentration called critical micelle concentration at which micelles are formed. for soaps, the
is to
New answer posted
3 months agoContributor-Level 9
- atom
or
or
Hence, number of different lines possible
Minimally, both can have same transition i.e etc.
New answer posted
3 months agoContributor-Level 9
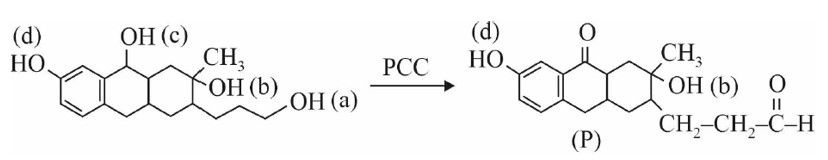
Nucleophilic addition on carbonyl compounds decreases by increasing steric hindrance.
New answer posted
3 months agoContributor-Level 9
Due to Intramolecular hydrogen bonding, O-nitrophenol fails to spread its surface, so that its b.p is less than para-nitrophenol.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers