Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
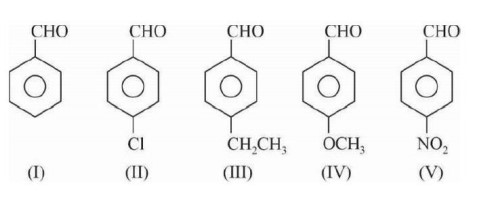
Key point: Fehling solution do not oxidise aromatic aldehydes, except when EWG present
at O/P position So (I), (III), (IV) not show this test
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
According to VSEPR theory:
For the species which contain
Has square pyramidal shape
These are BrF? , ClF? , XeOF?
New answer posted
3 months agoContributor-Level 10
Total number of electron in Ti = 22
Total number of electron in Ti? = 22 – 4 = 18 So EAN value of Ti = 18 + 12 + 4 = 34
New answer posted
3 months agoContributor-Level 10
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
New answer posted
3 months agoContributor-Level 10
Initial m equivalent of Cu²? = 200 * 0.5 * 2 = 200 m eq
So electricity passed = (0.965*3600)/96500 = 36 * 10? ³ eq. = 36 m eq
m eq CuBr? remaining = 200 – 36 = 164 ∴ N = meq / V (in ml) = 164/200 = 0.82
New answer posted
3 months agoContributor-Level 10
Mol wt of propane = 44 g and weight of propane = 12.0 g
So, using PV = nRT
V = (nRT)/P = (12/44) * 0.082 * 333) / (740/760) = 7.648 L
New answer posted
3 months agoContributor-Level 10
From ΔT? = K? * m, and ΔT? = 15°C
∴ m = ΔT? / K? = 15 / 1.86 = 8.06
So the amount of propyl alcohol to be added.
= m * molwt = 8.06 * 60 = 483.6 g
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers