Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
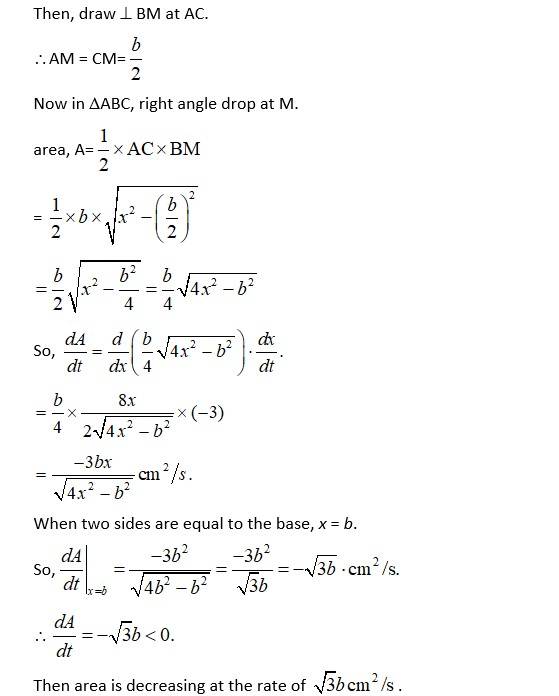
Let 'b' and 'x' be the fixed base and equal side of isosceales triangle.
Then, cm/s (Ø decreasing).
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The vapour pressure of a liquid in comparison to air pressure determines its boiling point. At a constant atmospheric pressure, the lower the vapour pressure, the higher the boiling point of a liquid, and vice versa.
Because NaCl is a nonvolatile solute, it reduces the vapour pressure of water when added to it. The boiling point of water rises as a result. Methyl alcohol, on the other hand, is more volatile than water, therefore adding it to the solution raises the overall vapour pressure, lowering the boiling point of water.
New answer posted
7 months agoContributor-Level 10
Because both components exist in the distillate and the liquid and vapour compositions are the same, this indicates that the liquids have formed an azeotropic combination that cannot be separated at this stage by fractional distillation.
New answer posted
7 months agoContributor-Level 10
We have, f(x)
f(x) =
f(x) =
=
= =
At extreme points, f(x) = 0.
At x = e, f"(e) =
x = e is a point of maximum.
New answer posted
7 months agoContributor-Level 10
(a) Consider
Now, is approximately equal to and is given by,
Hence, the approximate value of is
=0.677
(b)
(b) Consider
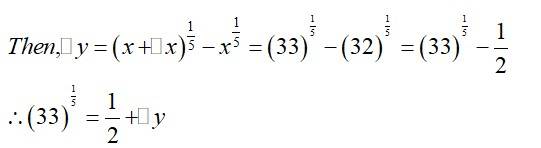
Now, is approximately equal to and is given by,
Hence, the approximate value of
is
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
When dissolved in suitable solvents, certain solutes/compounds either dissociate or associate. For instance, ethanoic acid dimerises in benzene due to hydrogen bonding, but dissociates and produces ions in water.
As a result, the number of chemical species in solution rises or falls in relation to the number of chemical species of solute used to create the solution. Because the magnitude of the colligative property is dependent on the number of solute particles in relation to the total number of particles in solution, it is expected that the molar mass calculated using c
New answer posted
7 months agoContributor-Level 10
We have,
At f(x) = 0.
2x – 1 = 0
=
Option (B) is
Hence maximum value of f(x) = at x = 1 and x = 0.
Option (c) is correct.
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
(i) When egg is placed in a dilute mineral acid solution (preferably dilute HCl solution), the hard external CaCO3 layer of the egg dissolves out /removed without damaging its semipermeable membrane.
(ii) Yes, this egg can be inserted into a bottle with a narrow neck without distorting in shape. The process involved utilising phenomenon of osmosis is explained as below -
Egg is placed in a mineral acid solution – after some time the egg is removed and placed in a hypertonic solution- size of the egg gradually decreases after some time and it shrivels due to osmos
New answer posted
7 months agoContributor-Level 10
We have,
At f(x) = 0.
x = 1 and x = -1.
At
At x = -1,
The maximum value of f(x)
Hence, option (D) is correct.
New answer posted
7 months agoContributor-Level 10
The equation of the given curve is
x2 = 2y.
Let p (x, y) be a point on the curve.
The distance of p (x, y) from (0, 5) is say S is given by
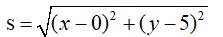
Let z = s2 = x2 + y2 + 25 – 10y = 2y + y2 -10y + 25
z = y2 – 8y + 25
So,
At
At y = 4,
y = 4, is point of minimum distance.
So, x2 = 2y->x2 = 2 * 4-> x2 = 8
Hence, the point of the nearest distance are and
Option (A) is correct.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers
