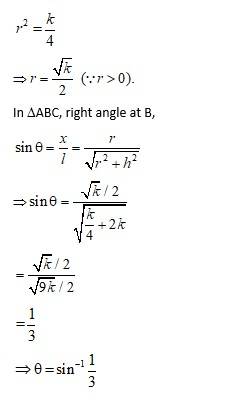Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
The process of osmosis is of immense biological and industrial importance as is evident from the following examples:
A. Biological Importance :
(i) Osmosis is responsible for some of the water movement from the soil into the plant roots and then into the top parts of the plant.
(ii) Adding salt to meat to protect it against bacterial action (ie. salting).
(iii) Sugar is used to protect fruits from bacterial attack. Bacterium in canned fruit shrivels and dies as a result of the osmosis process.
(iv) Salt blood cells break due to osmosis when placed in water containing les
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Raised increases in size when submerged in water. It's due to a phenomenon known as "Osmosis." The process is depicted graphically in figure. A semipermeable membrane separates a solution from its solvent in this procedure, allowing solvent molecules to pass through but preventing solute particles from passing through.
The passage of solvent molecules from a pure solvent to a solution via a semipermeable membrane is known as osmosis. The following are three osmosis applications:
(i) Osmosis is responsible for some of the water movement from the soil into the plant roots
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
"Azeotropes” is the general term for binary mixes that deviate from Rault's law and whose components cannot be separated by fractional distillation. Because of the following reasons, fractional distillation cannot produce pure ethanol: Azeotropes are binary solutions (liquid mixes) with the same composition in the liquid and vapour phases, therefore fractional distillation cannot separate the components of an azeotrope. On fractional distillation, an ethanol-water mixture (obtained via sugar fermentation) yields a solution containing approximately 95 percent ethanol b
New answer posted
7 months agoContributor-Level 10
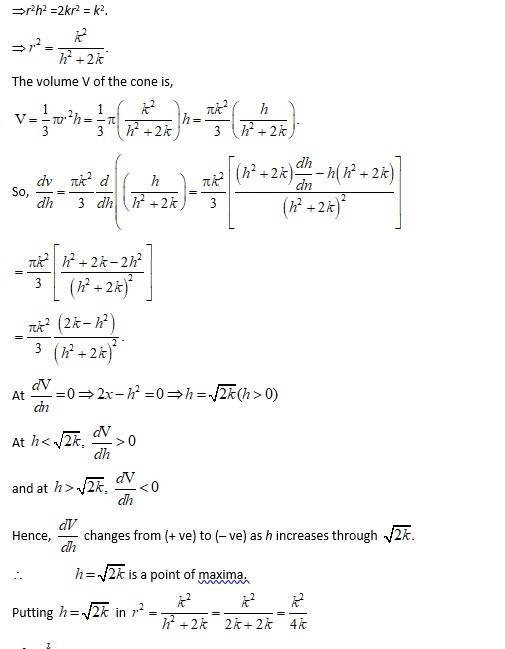
Let r, h, l and Ø be the radius, height, slant height and semi-vertical angle respectively of the cone. i.e., r, h, l>0.
Then, Volume V of the cone is
So,
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
Ideal solution: The 'ideal solution' is a binary solution of two volatile liquids that follows Raoult's rule at any concentration and temperature.
Ideal solutions are formed when the intermolecular attractive forces between the solute (A) and the solvent (B) (ie.A-B interaction) are approximately equal to those between the solvent-solvent (A-A) and the solute-solute (BB). Enthalpy of mixing, mixing H=0, in such a perfect solution.
Volume change on mixing, Δ mixing V=0.
Examples: n- hexane and n-heptane.
Non ideal solution: At any concentration and temperature, these
New answer posted
7 months agoContributor-Level 10
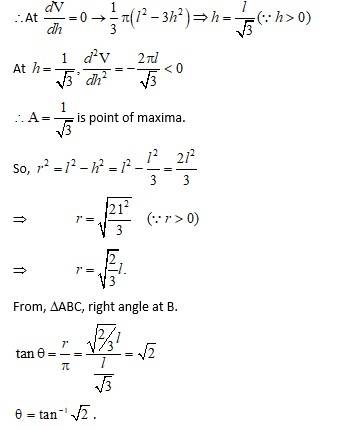
Let r and h be the radius and height of the cone.
The volume V of the cone is.
And curve surface area S is
New answer posted
7 months agoContributor-Level 10
Let r and h be the radius and height of the one in scribed in the sphere of radius R.
Then, is ΔOBC, rt angle at B (h-r)2 + r2 = R2
h2 + R2- 2hR + h2 = R2
r2 = 2hR -h2
Then the volume v of the cone is,
At
4Rh – 3h2 = 0.
h(4R – 3h) = 0.
h = 0 and
As h> 0,
At
is a point of maxima.
and
Hence, Volume of Cone,
Volume of sphere.
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
(i) For a binary solution having both components as volatile liquids (viz. CHCl3 and CH2Cl2), the total pressure will be
p=
p1= partial vapour pressure of component 1 (ie. CHCl3)
p2= partial vapour pressure of component 2 (ie. CH2Cl2)
(ii) For a solution containing non-volatile solute ie. NaCl (s) and H2O (l), the Raoult's law is applicable only to vaporisable component (1) ie. H2O (l) and total vapour pressure is written as
P= P1= x1P10+ x2P20
= x1P10+ (1-x1) P20
(P10-P20)X1 + P20
p=
p1= partial vapour pressure of component 1 (CHCl3 )
New answer posted
7 months agoContributor-Level 10
Let x and y in 'm' be the length of side of the square the radius of the circle respectily
Then, length of wire = perimeter of square + circumference of circle
28 = 4x + 2πy
2x + πy = 14
The combine area A of the square and the circle is
A = x2 + πy2
So,
At,
At,
isa point of minima
Hence, length of square =
and length of circle = 2πy
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers