Ncert Solutions Chemistry Class 11th
Get insights from 2k questions on Ncert Solutions Chemistry Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iii)
In free expansion, w=0 because volume is constant, as the process is adiabatic q = 0 and from first law of thermodynamics.
U=q+w
This means that internal energy remains constant.
New answer posted
5 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(i) The -CH3 group is an activating group and it directs the upcoming substituent to the ortho and para position.
New answer posted
5 months agoContributor-Level 10
This is a assertion and reason answer type question as classified in NCERT Exemplar
(i) Because cyclooctatetraene is tubbed shaped.
New answer posted
5 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
(i) → (d); (ii) → (a); (iii) → (b) : (iv) → (c)
New answer posted
5 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
(i) → (d); (ii) → (c); (iii) → (b) : (iv) → (a)
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
The correct option is B
The balanced equation for the combustion of methane is:
CH4 (g)+2O2 (g)→CO2 (g)+2H2O (l)
Here, Δng=1−3=−2
ΔH
=ΔU
+ΔngRT
ΔH
=−393−2RT
∴ΔH
New answer posted
5 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
(i) → (b); (ii) → (c); (iii) → (a)
New answer posted
5 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (iii)
Standard enthalpy of combustion is defined as the enthalpy change per mole (or per unit amount) of a substance when it undergoes combustion and all the reactants and products being in their standard states at the specified temperature.
New answer posted
5 months agoContributor-Level 10
This is a matching answer type question as classified in NCERT Exemplar
(i) → (d); (ii) → (a); (iii) → (e) : (iv) → (c) : (v) → (b)
New answer posted
5 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) & (iii)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 679k Reviews
- 1800k Answers

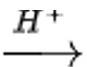 CH3CH2OH
CH3CH2OH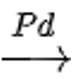 CH3CH3
CH3CH3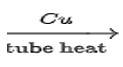 C6H6
C6H6