Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
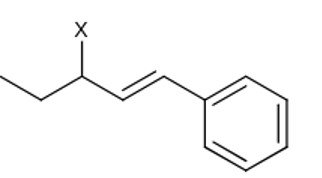
Allylic halide have halogen bonded to sp³ carbon which is adjacent to > C = C <
New answer posted
3 months agoContributor-Level 10
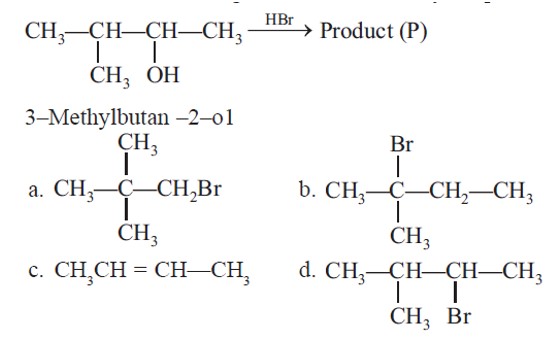
Initially, after protonation followed by loss of water, secondary carbocation is formed, further Hydride shift leads to 3° carbocation.
New answer posted
3 months agoContributor-Level 10
The greater stability of Cu²? (aq) rather than Cu? (aq) is due to the much more negative ΔhydH of Cu²? (aq) than Cu? , which more than compensates for the second ionisation enthalpy of Cu.
New answer posted
3 months agoContributor-Level 10
When the reactants and the catalyst are in different phases, then the catalysis is known as heterogenous catalysis.
1. N? (g) + 3H? (g) - (Fe (s)-> 2NH? (g) (Heterogenous catalysis)
2. 2SO? (g) + O? (g) - (NO (g)-> 2SO? (g) (Homogenous catalysis)
3. C? H? O? (aq) + H? O (l) → C? H? O? (aq) + C? H? O? (aq) (Homogenous catalysis)
4. NO (g) + O? (g) → NO? (g) + O? (g)
New answer posted
3 months agoContributor-Level 10
Since, atom B forms CCP structure. Therefore, there will be 4-B atoms.
Now, atom A occupies 1/3 of tetrahedral voids.
Hence, number of A atoms = 1/3 * 8 = 8/3
The correct formula of the compound = A? /? B?
= A? B?
x + y = 2 + 3 = 5
New answer posted
3 months agoContributor-Level 10
H? O < H? S < H? Se < H? Te
Down the group acidic strength increases
So pK? value decreases
New answer posted
3 months agoContributor-Level 10
n (O? ) = 4/32 = 1/8 mol
n (H? ) = 2/2 = 1 mol
n (Total) = n (O? ) + n (H? ) = 1/8 + 1 = 9/8 mol
PV = nRT
P (Total) * 1 = (9/8) * 0.082 * 273
P (Total) = 25.18 atm
New answer posted
3 months agoContributor-Level 10
Λ°? (CH? COOH) = Λ°? (H? ) + Λ°? (CH? COO? ) = 350 + 50 = 400Scm² mol? ¹
α = Λ? / Λ°?
α = 20/400 = 5 * 10? ²
K? (CH? COOH) = Cα²
= 0.007 * (5 * 10? ²)²
= 1.75 * 10? molL? ¹
New answer posted
3 months agoContributor-Level 10
R: CH? OH
Certain mild reducing agents like hypophosphorus acid or ethanol reduce diazonium salts to arene and themselves get oxidised to phosphorous acid and ethanal respectively.
New answer posted
3 months agoContributor-Level 10
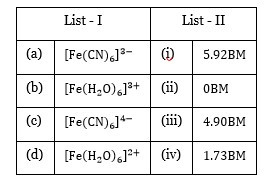
[Fe (CN)? ]? ³ Fe? ³ = 3d?
Unpaired electron = 1, μ = 1.7BM
[Fe (H? O)? ]? ³ Fe? ³ = 3d?
Unpaired electrons = 5, μ = 5.9BM
[Fe (CN)? ]? Fe? ² = 3d?
Unpaired electron = 0, μ = 0BM
[Fe (H? O)? ]? ² Fe? ² = 3d?
Unpaired electrons = 4, μ = 4.9BM
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers