Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Co (NH? )? ] [Cr (CN)? ]: Co-ordination isomerism
[Co (NH? )? (NO? )? ]: Linkage isomerism
[Cr (H? O)? ]Cl? : Solvate isomerism
Cis- [CrCl? (Ox)? ]³? : Optical isomerism
New answer posted
4 months agoContributor-Level 10
The change in entropy for the following processes is negative (ΔS = -ve), indicating an increase in order:
Water (l) → Ice (s) at 0°C
H? O (l) → Ice (s) at -10°C
N? (g) + 3H? (g) → 2NH? (g)
Adsorption
New answer posted
4 months agoContributor-Level 10
The reaction shown is:
Anisole diazonium chloride (A) + Ethanol → Anisole + Acetaldehyde (X) + HCl + N? (Y)
New answer posted
4 months agoContributor-Level 10
The reactions in the Solvay process are:
NaCl + H? O + NH? + CO? → NH? Cl + NaHCO?
2NaHCO? → Na? CO? + CO? + H? O
2NH? Cl + Ca (OH)? → CaCl? + 2NH? + H? O
CaCl? is a byproduct of the process.
New answer posted
4 months agoContributor-Level 9
o Chlorophyll: Magnesium present in chlorophyll.
o Vitamin B? : Cobalt (cyanocobalamin).
o Anticancer drug: Platinum (Coordination compound of platinum).
o Grubbs catalyst: Ruthenium.
New answer posted
4 months agoContributor-Level 10
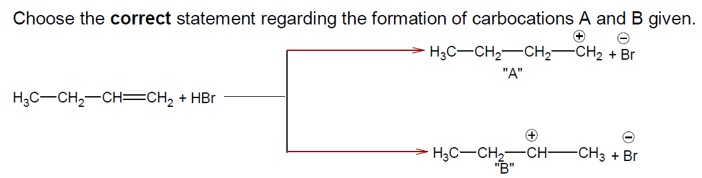
The addition of HBr to H? C−CH? −CH=CH? proceeds via carbocation formation.
Formation of a 1° Carbocation (A): H? C−CH?
Formation of a 2° Carbocation (B): H? C−CH? −C? H−CH?
The 2° carbocation (B) is more stable than the 1° carbocation (A). Therefore, the activation energy (Ea) for the formation of B is lower, and B is formed faster.
New answer posted
4 months agoContributor-Level 9
o Alcoholic potassium hydroxide (Alc KOH):- used for β - elimination.
o Pd / BaSO? : - Lindlar's Catalyst.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers