Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 9
Matching Processes with Catalysts:
o (a) Deacon's process: CuCl? used as catalyst
o (b) Contact process: V? O? used as catalyst
o (c) Cracking of hydrocarbon: ZSM-5 used as catalyst
o (d) Hydrogenation of vegetable oil: Particles Ni used as catalyst
New answer posted
4 months agoContributor-Level 9
Matching Compounds to their Class:
o Antacid: Cimetidine
o Artificial Sweetener: Alitame
o Antifertility: Novestrol
o Tranquilizers: Valium
New answer posted
4 months agoContributor-Level 9
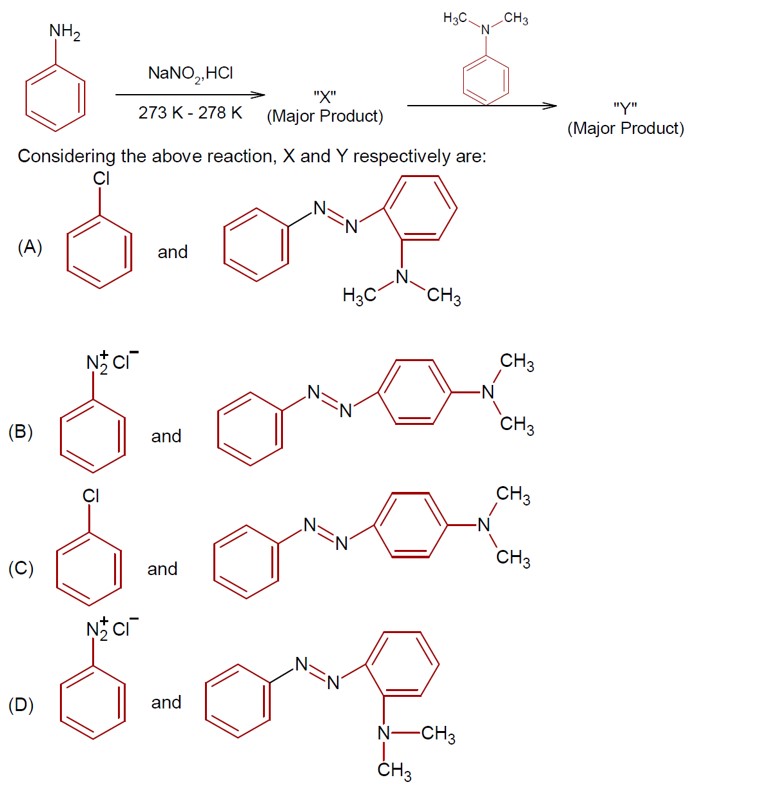
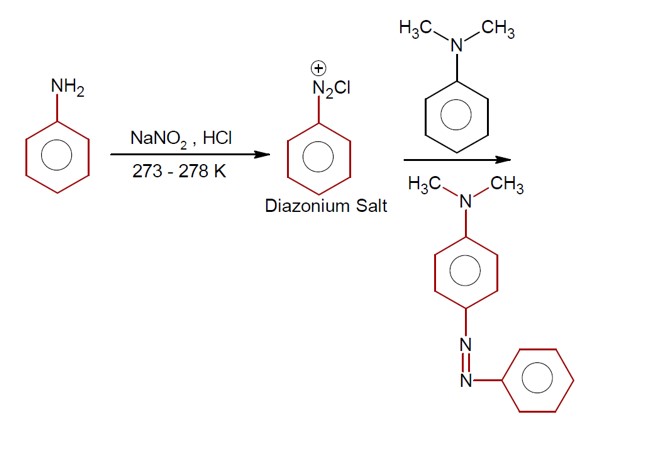
Reaction Sequence:
o NO? + CH? COOH → HNO? + CH? COO?
o Sulphanilic acid (+ NH? CH? COO? ) + HNO? → N-acetylated diazonium intermediate + 2H? O
The intermediate + another molecule → Red Azodye + NH? + CH? COOH
New answer posted
4 months agoContributor-Level 10
Eutrophication is the process in which a water body becomes overly enriched with nutrients, leading to the plentiful growth of simple plant life.
New question posted
4 months agoNew question posted
4 months agoNew answer posted
4 months agoContributor-Level 10
Unimolecular Reactions might seem to not participate in the collision theory due to the presence of only a single molecule. In such cases, the molecule is activated by external forces such as heat, light, electricity, or by colliding with the walls of the container. This force charges the molecule enough to break the activation barrier and result in an effective collision.
New answer posted
4 months agoContributor-Level 10
100 mol of KBr is doped with 10? mol of SrBr?
Therefore, 1 mol of KBr contains 10? mol of SrBr?
Molar mass of KBr = 119 g/mol .
119 g of KBr contains 10? mol of SrBr?
1 g of KBr contains (10? / 119) mol of SrBr?
Each Sr²? ion introduced creates one cation vacancy to maintain electrical neutrality.
Number of cation vacancies = (moles of SrBr? ) * (Avogadro's number)
Number of vacancies = (10? / 119) * (6.023 * 10²³) = 5.06 * 10¹?
The answer provided in the document is "5 (Rounded off)", which likely refers to the coefficient 5.06.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers