Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Molality = (mole of solute * 1000) / wt of solvent (gm)
100 = (n_solute * 1000) / [ (1 - n_solute) * 18]
(1 - n_solute) / n_solute = 1000 / (100 * 18) = 10/18
18 (1 - n_solute) = 10 n_solute
18 - 18 n_solute = 10 n_solute
18 = 28 n_solute
n_solute = 18 / 28? 0.6428 = 64.28 * 10? ²
Ans = 64 (Rounded off)
New answer posted
4 months agoContributor-Level 10
For n = 4, the possible values of l are 0, 1, 2, 3.
For l = 3, and m = -3.
Radial nodes = (n - l - 1) = (4 - 3 - 1) = 0
Ans = 0
New answer posted
4 months agoContributor-Level 10
Cr? O? ²? + Fe²? - (H? )-> Cr³? + Fe³?
(n=6) (n=1)
Meq Cr? O? ²? = Meq Fe²?
20 * 0.03 * 6 = 15 * M * 1
M = (20 * 0.03 * 6) / 15 = 0.24 = 24 * 10? ²
Ans = 24
New answer posted
4 months agoContributor-Level 10
P? + 3NaOH + 3H? O → PH? + 3NaH? PO? (A)
Here, NaH? PO? is a reducing agent.
NaH? PO? + AgNO? + H? O → Ag + HNO? + NaH? PO?
Equivalents of NaH? PO? = equivalents of Ag (n-factor of NaH? PO? = 4)
1 mole * 4 = n moles of Ag * 1
So, moles of Ag = 4.
New answer posted
4 months agoContributor-Level 10
For the weak acid HA in the presence of strong acid HCl:
Ka = [ (Cα + 0.1) * Cα] / [C (1-α)] ≈ (0.1 * 10? ²α) / 10? ² = 0.1α
Given Ka = 2 * 10?
2 * 10? = 10? ¹ * α
α = 2 * 10?
Ans = 2
New answer posted
4 months agoContributor-Level 10
Mole of CH? = 6.4 / 16 = 0.4 and mole of CO? = 8.8 / 44 = 0.2
Total mole = (0.4 + 0.2) = 0.6 mole of a non-reacting mixture of gas
Using Ideal Gas Law; P = nRT / V
P = (0.6 * 8.314 * 300) / 10 = 149.65 kPa
Ans = 150 (Rounded off)
New answer posted
4 months agoContributor-Level 10
Kt = 2.303 log? (A? /A? )
K * 570 = 2.303 log? (100/32)
K = [2.303 / 570] [log?10² - log?2? ]
K = [2.303 / 570] [2 - 5 * 0.301] = [2.303 / 570] * 0.495 = 0.002 = 2.0 * 10? ³ s? ¹
Ans = 2
New answer posted
4 months agoContributor-Level 10
Partial Pressure of O? = K? * solubility (K? = Henry's constant)
Solubility = PO? / K? = 20 / (8.0 * 10? ) = 2.5 * 10? = 25 * 10? M
Ans = 25
New answer posted
4 months agoContributor-Level 10
In an aromatic compound, overall delocalization is processed by the electrons according to Hückel's rule. For the compound in question, the total number of delocalized electrons is 6.
New answer posted
4 months agoContributor-Level 10
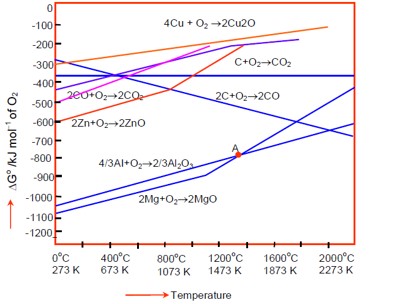
At the point of intersection in an Ellingham diagram? G for two processes becomes equal, so? G for the reduction becomes zero. A sudden increase in the slope indicates a change in the state of the metal oxide, i.e., from solid to liquid or liquid to vapor.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers