Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
The reaction is N? O? (g)? 2NO? (g).
Δn_g = (moles of gaseous products) - (moles of gaseous reactants) = 2 - 1 = 1.
The relationship between Kp and Kc is Kp = Kc (RT)^Δn_g.
600.1 = 20.4 * (0.0831 * T)¹
T = 600.1 / (20.4 * 0.0831) = 353.99 K.
The answer, rounded off, is 354 K.
New answer posted
4 months agoContributor-Level 10
Using the Ideal Gas Law, PV = nRT:
P = 1 bar
V = 20 mL = 0.020 L
R = 0.083 L·bar·mol? ¹·K? ¹
T = 273 K
n = PV / RT = (1 * 0.020) / (0.0831 * 273) = 8.8 * 10? mol of Cl?
Number of Cl? molecules (N) = n * N_A = (8.8 * 10? ) * (6.022 * 10²³) = 5.3 * 10²? molecules.
Number of Cl atoms = 2 * (5.3 * 10²? ) = 1.06 * 10²¹.
The answer, rounded off to the nearest integer for the power of 10²¹, is 1.
New answer posted
4 months agoContributor-Level 10
For the dissociation of K? [Fe (CN)? ]? 4K? + [Fe (CN)? ]? , the number of ions produced (n) is 5.
The degree of dissociation (α) is related to the van't Hoff factor (i) by α = (i-1)/ (n-1).
Given α = 0.4: 0.4 = (i - 1) / (5 - 1) => 1.6 = I - 1 => I = 2.6
Using the depression in freezing point formula (ΔTf = i·Kf·m), and equating the ΔTf for two different solutions:
ΔTf (K? [Fe (CN)? ]) = ΔTf (A)
i? ·Kf·m? = i? ·Kf·m?
(2.6) * [ (18.1 / M) / (100-18.1)/1000] = (1) * [ (w? /M? ) / (W? /1000)]
The problem simplifies to finding the molar mass M of solute A:
2.6 * [18.1 / (M * 81.9)] * 1000 = [w? /M? ] * [1000/W? ]
Assuming the secon
New answer posted
4 months agoContributor-Level 10
The reaction is: FeCl? + 3H? C? O? + 3KOH → K? [Fe (C? O? )? ] (A) + 3HCl + 3H? O
In the complex [Fe (C? O? )? ]³? , the oxalate ion (C? O? )²? is a bidentate ligand.
There are three bidentate ligands, so the coordination number, or secondary valency, of Fe is 3 * 2 = 6.
New answer posted
4 months agoContributor-Level 10
The cell constant (G) is given by G = κ * R, where κ is conductivity and R is resistance.
Since the cell constant is constant: (κ * R)KCl = (κ * R)HCl
0.14 Sm? ¹ * 4.19 Ω = κ_HCl * 1.03 Ω
κ_HCl = (0.14 * 4.19) / 1.03 = 0.569 Sm? ¹
This is equivalent to 56.9 * 10? ² Sm? ¹.
The answer, rounded off, is 57.
New answer posted
4 months agoContributor-Level 10
-SO? H acts as a cation exchanger.
-NH? acts as an anion exchanger.
New answer posted
4 months agoContributor-Level 10
3CaO + 2Al → 3Ca + Al? O?
ΔH? = ΣΔH? (Products) - ΣΔH? (Reactants)
ΔH? = [ (0) + (-1675) ] - [ (3 * -635) + (0) ]
ΔH? = -1675 - (-1905) = -1675 + 1905 = 230 kJ
Ans = 230
New answer posted
4 months agoContributor-Level 10
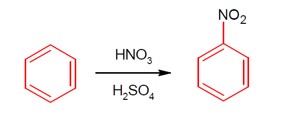
Moles of benzene = 3.9 g / 78 g/mol . This would produce (3.9 / 78) moles of nitrobenzene in 100% conversion.
Produced moles of nitrobenzene = 4.92 g / 123 g/mol .
% yield = [ (4.92 / 123) / (3.9 / 78) ] * 100 = [ (4.92 * 100 * 78) / (123 * 3.9) ] = 80.0%
Ans = 80
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers